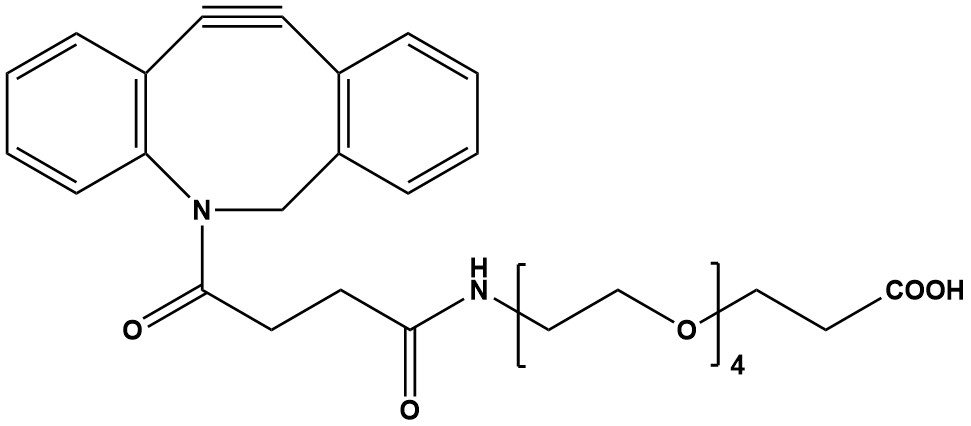
| Synonym: | DBCO-CONH-PEG4-CH2CH2COOH |
| CAS #: | 1537170-85-6 |
| Molecular Formula: | C30H36N2O8 |
| Molecular Weight: | 552.6 |
| DBCO-PEG4-acid, also known by synonyms such as Dibenzocyclooctyne-PEG4-acid or Polyethylene glycol, DBCO-PEG4-Acid, is a highly versatile and increasingly important reagent in the fields of chemical biology, materials science, and drug discovery. It is a heterobifunctional linker that combines the highly reactive dibenzocyclooctyne (DBCO) moiety with a hydrophilic polyethylene glycol (PEG) spacer and a terminal carboxylic acid group. This unique architecture makes it a powerful tool for a wide array of applications, particularly in “copper-free” click chemistry and bioconjugation. 1. Chemical Structure and Properties DBCO-PEG4-acid features three key components: 1.1 Dibenzocyclooctyne (DBCO) Group This strained alkyne moiety is the cornerstone of its reactivity. The inherent ring strain of the cyclooctyne allows it to react rapidly and efficiently with azide-functionalized molecules via a [3+2] cycloaddition reaction, known as Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC). Critically, this reaction occurs without the need for a copper(I) catalyst, which is often cytotoxic and can interfere with biological systems. This “copper-free click chemistry” is a significant advantage in sensitive biological applications. 1.2 PEG4 Spacer The “PEG4” refers to a short polyethylene glycol chain consisting of four ethylene glycol units. PEG spacers are renowned for their hydrophilicity and flexibility. • Increased Water Solubility: The PEG chain significantly enhances the water solubility of the overall molecule, which is crucial for reactions in aqueous biological buffers. • Reduced Aggregation and Steric Hindrance: The flexible PEG linker minimizes non-specific interactions and aggregation, and provides a “spatial arm” between the DBCO and carboxylic acid functionalities. This reduces steric hindrance, allowing for more efficient and specific conjugation reactions with larger biomolecules. • Improved Biocompatibility: PEGylation is a well-established strategy to improve the pharmacokinetics and reduce the immunogenicity of conjugated biomolecules, making PEGylated compounds more suitable for in vivo applications. 1.3 Carboxylic Acid Group The terminal carboxylic acid (−COOH) is a highly versatile reactive handle. It can be easily activated (e.g., using carbodiimides like EDC or HATU in combination with NHS) to form an activated ester or other electrophilic species, which can then react with primary amines (−NH2) present on proteins, peptides, small molecules, or surfaces, forming stable amide bonds. 2. Key Applications The unique combination of a bioorthogonal DBCO group, a hydrophilic PEG spacer, and a reactive carboxylic acid allows DBCO-PEG4-acid to be employed in a wide range of applications: • Bioconjugation: This is the primary application. DBCO-PEG4-acid serves as a versatile linker to conjugate a variety of molecules (e.g., peptides, proteins, antibodies, nucleic acids, nanoparticles, fluorescent dyes) to azide-modified targets. The reaction is specific, efficient, and occurs under mild physiological conditions, preserving the integrity and biological activity of sensitive biomolecules. • PROTAC Synthesis: Emerging research highlights its use as a PEG-based PROTAC (PROteolysis TArgeting Chimeras) linker. PROTACs are heterobifunctional molecules designed to induce the degradation of target proteins by bringing them into proximity with E3 ubiquitin ligases. The DBCO-PEG4-acid can connect an E3 ligase ligand to a target protein ligand, enabling the development of novel targeted therapeutic agents. • Protein Labeling and Modification: It can be used to introduce a DBCO handle onto proteins or other amine-containing biomolecules. This modified biomolecule can then be reacted with azide-functionalized probes (e.g., fluorescent dyes, biotin, or other reporters) for various detection, imaging, or immobilization purposes. • Drug Delivery Systems: The compound is instrumental in the development of targeted drug delivery systems. Drugs or imaging agents can be conjugated to antibodies, nanoparticles, or other carriers via the DBCO-PEG4-acid linker, allowing for site-specific delivery and reduced off-target effects. • Surface Modification: The carboxylic acid can be used to functionalize surfaces (e.g., nanoparticles, biosensors, microarrays) with the DBCO moiety, enabling the subsequent immobilization of azide-tagged biomolecules for diagnostic or material science applications. • Synthesis of Complex Bioconjugates: It acts as a crucial building block for creating more complex architectures, such as branched or multi-functional linkers, by allowing further modifications at the carboxylic acid end after initial DBCO-azide click. 3. Advantages • Copper-Free Reaction: Eliminates the need for cytotoxic copper catalysts, making it ideal for in vitro and in vivo biological applications where cell viability and integrity are paramount. • High Specificity and Efficiency: The SPAAC reaction is highly specific for azides and DBCOs, ensuring minimal side reactions with endogenous biological functionalities (like amines, thiols, or hydroxyls). The reaction kinetics are fast, leading to high yields of stable triazole linkages. • Excellent Biocompatibility: The PEG spacer enhances water solubility and reduces non-specific binding, aggregation, and immunogenicity, crucial for biological research and therapeutic development. • Versatile Amine-Reactive Handle: The terminal carboxylic acid allows for conjugation to a wide array of amine-containing molecules via well-established amide coupling chemistries. • Thermal Stability: The DBCO group itself is thermally stable, ensuring reliable conjugation under various conditions. • Modular Design: Its heterobifunctional nature allows for a stepwise and controlled conjugation strategy, enabling the synthesis of well-defined conjugates. 4. Considerations and Best Practices • Solubility: While the PEG chain enhances aqueous solubility, DBCO-PEG4-acid itself may initially require dissolution in water-miscible organic solvents (e.g., DMSO, DMF, DCM) before dilution into aqueous buffers for reactions. • Storage: Like many reactive chemical reagents, it should be stored under appropriate conditions (typically at -20°C, desiccated, and protected from light) to maintain purity and reactivity, as the acid group can be sensitive to moisture. • Reaction Conditions: Amide bond formation from the carboxylic acid typically requires activators (EDC, HATU, DCC) and can be optimized by adjusting pH and reagent concentrations. The SPAAC reaction is generally robust across a range of physiological pH values. • Analytical Characterization: Proper characterization of the conjugated product (e.g., using mass spectrometry, HPLC, SDS-PAGE) is crucial to confirm successful conjugation and determine the conjugation efficiency. DBCO-PEG4-acid stands out as a powerful and indispensable tool in modern chemical biology and biotechnology. Its ability to facilitate highly specific, efficient, and biocompatible copper-free click reactions, combined with the beneficial properties of the PEG spacer and a versatile carboxylic acid handle, makes it a go-to reagent for a broad spectrum of bioconjugation strategies, from fundamental research to the development of advanced therapeutics and diagnostics. As research in areas like targeted drug delivery and protein degradation continues to expand, DBCO-PEG4-acid is poised to remain a critical component in the synthetic chemist’s toolkit. References 1. Overview of Click Chemistry 2. Click chemistry 3. Copper-free click chemistry 4. Introduction: Click Chemistry 5. Click Chemistry Azide-Alkyne Cycloaddition |
|
DBCO-PEG4-acid
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
Spermine(N3BBB)
-
1,3,4,6-Tetra-O-β-acetyl-N-azidoacetylmannosamine
-
Spermine(N3HHH)•3HCl
-
DBCO-PEG6-acid
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-α-D-glucopyranose
-
2-Azidoethyl 2,3,4,6-Tetra-O-acetyl-β-D-galactopyranoside
-
N-Azidoacetyl-β-D-glucosamine tetraacetate
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-α-D-galactopyranose
-
2,3,4,6-Tetra-O-acetyl-β-D-mannopyranosyl Azide