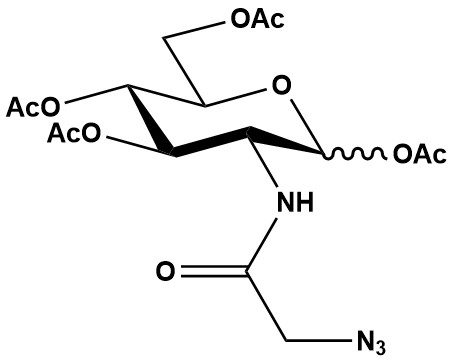
| Synonym: | N-azidoacetylglucosamine-tetraacylated |
| CAS #: | 98924-81-3 |
| Molecular Formula: | C16H22N4O10 |
| Molecular Weight: | 430.4 |
| Ac4GlcNAz (N-Azidoacetylglucosamine-tetraacetylated) is a widely used metabolic chemical reporter (MCR) in glycobiology and chemical biology, primarily employed to study O-linked β-N-acetylglucosamine (O-GlcNAc) modifications and N-linked glycosylation. By introducing an azide handle into cellular glycans, Ac4GlcNAz facilitates subsequent bioorthogonal “click chemistry” for visualization, enrichment, and identification of relevant glycoconjugates. 1. Mechanism of Action and Metabolic Labeling The functional mechanism of Ac4GlcNAz follows the general principles of metabolic glycoengineering: 1.1 Cell Permeability The peracetylated form of GlcNAz (Ac4GlcNAz) is lipophilic, allowing it to efficiently diffuse across the cell membrane into the intracellular compartment. 1.2 Intracellular Deacetylation Once inside the cell, ubiquitous intracellular carboxyesterases remove the acetyl groups, liberating the free N-azidoacetylglucosamine (GlcNAz). 1.3 Metabolic Incorporation The deacetylated GlcNAz then enters the hexosamine biosynthetic pathway. It acts as an analog of natural N-acetylglucosamine (GlcNAc). • O-GlcNAcylation: GlcNAz can be converted to UDP-GlcNAz and then serve as a substrate for O-GlcNAc transferase (OGT), an enzyme that adds O-GlcNAc to serine and threonine residues of nuclear and cytoplasmic proteins. This makes Ac4GlcNAz a tool for studying O-GlcNAc modifications. • N-linked Glycosylation: GlcNAz can also enter the N-linked glycosylation pathway, though its incorporation into mature N-glycans on the cell surface might be less prominent or more complex due to pathway intermediates and competition with endogenous sugars. The hexosamine biosynthetic pathway ultimately leads to the formation of UDP-GlcNAc, which is a key donor for both O-GlcNAcylation and N-linked glycosylation. 1.4 Bioorthogonal Ligation (Click Chemistry) The azide group introduced into the cellular glycoconjugates (proteins or lipids) acts as a unique chemical handle for subsequent bioorthogonal reactions: • Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC): This highly efficient reaction requires a copper(I) catalyst but the cytotoxicity of copper can be a limiting factor for live-cell studies. • Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) or Copper-Free Click Chemistry: This method utilizes strained cyclooctynes (e.g., DBCO, BCN) that react with azides without a metal catalyst, making it preferable for live-cell and in vivo applications. • Through these reactions, a reporter molecule (e.g., fluorescent dye, biotin, affinity tag) containing an alkyne or cyclooctyne can be specifically attached to the azide-labeled biomolecules. 2. Applications Ac4GlcNAz plays a significant role in various areas of glycobiology and chemical biology, particularly in studying O-GlcNAcylation: 2.1 Study of O-GlcNAcylation This is a key application. O-GlcNAcylation is a dynamic and reversible post-translational modification on nuclear and cytoplasmic proteins, akin to phosphorylation, regulating numerous cellular processes. Ac4GlcNAz allows: • Visualization: Fluorescent labeling of O-GlcNAc-modified proteins for detection by microscopy, flow cytometry, or in-gel fluorescence. • Proteomic Identification: Enrichment and identification of O-GlcNAc-modified proteins using biotinylated probes followed by mass spectrometry. • Functional Studies: Investigating the role of O-GlcNAc in cell signaling, metabolism, gene expression, and disease states (e.g., cancer, diabetes, neurodegeneration). 2.2 N-linked Glycosylation Studies While often overshadowed by its use for O-GlcNAc, GlcNAc is the foundational sugar for N-linked glycosylation, and Ac4GlcNAz can also be incorporated into these pathways, although specificity can be a challenge. 2.3 Cell Surface Engineering The introduction of azide tags onto cell surface glycoproteins can be used to engineer cell surfaces for various applications, such as targeted drug delivery or cell adhesion modulation. 2.4 Drug Discovery and Target Validation Identifying and characterizing O-GlcNAc-modified proteins can reveal novel drug targets or pathways implicated in disease. 2.5 GlycoRNA Discovery Recent groundbreaking research has shown that Ac4GlcNAz, along with other azidosugars, can be incorporated into glycoRNA, a novel class of RNA that is covalently modified with carbohydrates, primarily found in exosomes. This opens up entirely new avenues for understanding RNA modifications and their biological roles. 3. Advantages • Bioorthogonality: The azide group is largely inert in biological systems, ensuring highly specific labeling with minimal background noise. • Cell Permeability: The acetyl groups facilitate efficient cellular uptake, making it suitable for live-cell experiments. • Non-Radioactive: Offers a safer and more convenient alternative to traditional radioisotope labeling methods for studying glycosylation. • Versatility: Compatible with both CuAAC and SPAAC/copper-free click chemistry, allowing flexibility depending on the experimental requirements (e.g., in vitro lysate vs. live-cell imaging). 4. Challenges and Limitations Despite its utility, Ac4GlcNAz has some specific limitations, especially concerning specificity for O-GlcNAc and labeling efficiency: 4.1 Metabolic Cross-talk and Specificity • A significant challenge with Ac4GlcNAz is its relatively lower labeling efficiency for O-GlcNAc compared to other azidosugars, notably Ac4GalNAz. This is due to the UDP-GlcNAc pyrophosphorylase step in the GlcNAc salvage pathway being rate-limiting for UDP-GlcNAz biosynthesis. • Furthermore, Ac4GalNAz (N-azidoacetylgalactosamine-tetraacetylated) has been shown to be more effective for labeling O-GlcNAcylated proteins than Ac4GlcNAz itself. This surprising observation is due to metabolic cross-talk: Ac4GalNAz is deprotected to GalNAz, converted to UDP-GalNAz, and then epimerized by UDP-Glc/GalNAc 4-epimerase (GALE) into UDP-GlcNAz, which then efficiently labels O-GlcNAc. This means that if the goal is to label O-GlcNAc, Ac4GalNAz might paradoxically be a better choice in some systems, though it comes with the caveat of also labeling mucin-type O-glycans. • This metabolic interconversion can complicate the interpretation of results, as an azide signal obtained with Ac4GlcNAz may not solely represent O-GlcNAc, and an azide signal from Ac4GalNAz may not solely represent mucin-type O-glycans. 4.2 Toxicity of Copper Catalyst (for CuAAC) As with other azidosugars, if CuAAC is used, the inherent toxicity of copper ions limits its application in live-cell or in vivo settings, necessitating copper-free click chemistry. 4.3 Concentration Optimization The optimal concentration for labeling can vary significantly between cell types and experimental conditions. High concentrations could lead to off-target effects or perturb cellular metabolism. Typical concentrations are often in the 25-75 μM range for cell labeling. 4.4 Competition with Endogenous Sugars The efficiency of metabolic incorporation can be influenced by the intracellular pool of endogenous GlcNAc, which competes with GlcNAz for enzymatic incorporation. 5. Recent Research Advancements Research continues to address the limitations and expand the applications of azidosugars like Ac4GlcNAz: • Improved Reporter Design: The understanding of metabolic cross-talk has led to the development of “designer” azidosugars (e.g., 4-deoxy-4-fluoro-GlcNAz) that aim for higher specificity by resisting epimerization or by enhancing substrate affinity for specific enzymes. • Metabolic Engineering for Enhanced Labeling: Strategies to overcome metabolic bottlenecks, such as overexpression of specific enzymes in the hexosamine biosynthetic pathway (e.g., engineered GlcNAc kinase or UDP-GlcNAc pyrophosphorylase), have been explored to increase the efficiency of azidosugar incorporation. • Combined Approaches: Integrating Ac4GlcNAz labeling with other techniques, such as chemoenzymatic labeling (using engineered glycosyltransferases to add specific tags), or advanced mass spectrometry, enhances the depth and specificity of O-GlcNAc analysis. • Exploration of GlycoRNA: The recent discovery of glycoRNA and the role of azidosugars, including Ac4GlcNAz, in its labeling, highlights a new frontier in the study of RNA modifications and their functional implications. Ac4GlcNAz is a valuable tool for investigating O-GlcNAcylation, a critical and widespread post-translational modification. While its direct labeling efficiency for O-GlcNAc might be surpassed by other azidosugars (like Ac4GalNAz due to metabolic cross-talk), its role in understanding the hexosamine biosynthetic pathway and its newly discovered involvement in glycoRNA underscore its continued importance in the field of chemical glycobiology. References: 1. Metabolic glycan labelling with bio-orthogonal targeting and its potential in drug delivery 2. Metabolic glycan labeling and chemoselective functionalization of native biomaterials 3. Metabolic cross-talk allows labeling of O-linked β-N-acetylglucosamine-modified proteins via the N-acetylgalactosamine salvage pathway 4. A metabolic labeling approach toward proteomic analysis of mucin-type O-linked glycosylation 5. Overview of Click Chemistry |
|
Ac4GlcNAz
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
DBCO-PEG8-acid
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-D-glucopyranose
-
Ac4GlcNAlk
-
Spermine(N3HHH)•3HCl
-
2,3,4,6-Tetra-O-acetyl-β-D-mannopyranosyl Azide
-
2-Azidoethyl 2,3,4,6-Tetra-O-acetyl-β-D-galactopyranoside
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-β-D-galactopyranose
-
DBCO-PEG12-acid
-
2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl Azide