| Synonym: | tri-Boc-azido-spermine |
| CAS #: | 1190203-80-5 |
| Molecular Formula: | C25H48N6O6 |
| Molecular Weight: | 528.7 |
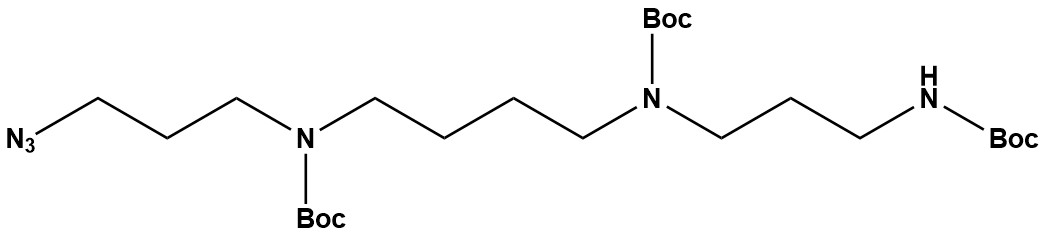
| Spermine(N3BBB) is a highly specialized, protected, and functionalized derivative of the naturally occurring polyamine, spermine. Unlike Spermine(HBBB) (Tris-Boc-spermine), which features three Boc protecting groups and one free amine, Spermine(N3BBB) incorporates an azide group along with three Boc protecting groups. This unique combination makes it an exceptionally valuable building block for click chemistry applications, particularly in bioconjugation and advanced materials science. 1. Chemical Structure and Properties • Core Structure: The fundamental skeleton is spermine (N1,N4-Bis(3-aminopropyl)butane-1,4-diamine), a linear polyamine with four amine groups. • Three tert-Butyloxycarbonyl (Boc) groups: These are acid-labile protecting groups commonly used for amines. Their presence on three of the four spermine amine groups provides control over reactivity, allowing for selective functionalization. • One Azide group (-N3): This is the defining feature of Spermine(N3BBB). The azide group is typically introduced onto one of the terminal primary amines of the spermine backbone (after appropriate selective protection). The azide group is highly stable under physiological conditions but is exceptionally reactive in click chemistry reactions, particularly Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC) and Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC). • Solubility: Expected to be soluble in common organic solvents like DCM, chloroform, DMSO, and ethyl acetate due to the lipophilic Boc groups. • Storage: Generally recommended to store at 2-8 °C or -20 °C, protected from light and moisture, to maintain product integrity and prevent degradation of the azide group. 2. Applications Spermine(N3BBB) is a highly specialized reagent predominantly used in click chemistry to create complex bioconjugates and advanced materials. Its core applications are centered around its azide functionality and the underlying polyamine scaffold. 2.1 Click Chemistry Bioconjugation • Copper(I)-catalyzed Azide-Alkyne Cycloaddition (CuAAC): The “gold standard” click reaction. Spermine(N3BBB) can be efficiently conjugated to molecules containing an alkyne (or terminal alkyne) group (e.g., proteins, peptides, nucleic acids, nanoparticles, polymers) under mild conditions. • Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC): Also known as copper-free click chemistry. It reacts with strained alkynes (e.g., cyclooctynes like DBCO or BCN) without the need for a copper catalyst, which is vital for in vivo or cell-based applications where copper can be toxic. • Targeted Drug Delivery: Attaching spermine (and its beneficial properties) to targeting ligands (e.g., antibodies, peptides) for specific delivery of drugs, radionuclides, or genetic material to cells or tissues. • Molecular Imaging Probes: Incorporating spermine into imaging agents to study polyamine metabolism or for targeted imaging of specific cellular processes. • Immunology: Creating novel immunoconjugates for vaccine development or immunomodulation. • Diagnostics: Developing biosensors or diagnostic assays based on polyamine interactions. 2.2 Gene Delivery Systems • Polyamines are known to facilitate the condensation and delivery of nucleic acids (DNA, RNA) due to their cationic nature. Spermine(N3BBB) can be integrated into novel cationic lipids, polymers, or nanoparticles designed for gene therapy. The Boc groups are often removed after formulation or uptake to expose the multiple positive charges, enhancing interaction with nucleic acids and facilitating endosomal escape. The azide group allows for the facile attachment of additional targeting ligands or functionalities to these gene delivery vehicles. 2.3 Materials Science and Nanotechnology • Functionalized Nanomaterials: The azide group enables the surface functionalization of nanoparticles (e.g., gold nanoparticles, polymeric nanoparticles) with spermine, creating novel materials for drug delivery, sensing, or catalysis. • Hydrogels and Polymers: Spermine(N3BBB) can be used as a cross-linking agent or a monomer in the synthesis of advanced polymeric materials, where the polyamine structure can confer specific properties (e.g., pH responsiveness, metal binding). 2.4 Medicinal Chemistry Research • Polyamine Analogs: As a specifically protected spermine derivative, it is invaluable for synthesizing a new generation of polyamine analogs with tailored biological activities, often incorporating targeting moieties. • Probe Synthesis: Developing chemical probes to study polyamine-related enzymes or pathways. 3. Advantages • Click Chemistry Versatility: The azide group provides an extremely powerful and efficient handle for conjugation, compatible with various click chemistry partners (alkynes, strained alkynes). • Orthogonal Reactivity: The azide group is orthogonally reactive to the Boc-protected amines. This means the azide can be reacted without affecting the Boc-protected amines, which can then be deprotected later to expose the polyamine’s inherent charge and reactivity. • Spermine Scaffold Benefits: Inherits the biological relevance of the spermine scaffold, allowing the creation of conjugates that can interact with DNA, RNA, or polyamine-binding proteins. • Modular Synthesis: Facilitates a modular approach to synthesizing complex bioconjugates and functional materials. Spermine(N3BBB) stands as a highly advanced and indispensable building block in modern chemical biology and materials science. By ingeniously combining the biologically relevant polyamine scaffold with the powerful and versatile azide handle for click chemistry, it empowers researchers to construct sophisticated bioconjugates and functionalized materials with unprecedented control and efficiency. Its role in advancing targeted drug delivery, molecular imaging, gene therapy, and the creation of novel biomaterials underscores its significance as a cutting-edge reagent in the pursuit of innovative scientific and medical solutions. References 1. Spermine |
|
Spermine(N3BBB)
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
DBCO-PEG3-acid
-
3,4,6-Tri-O-acetyl-2-azido-2-deoxy-D-galactopyranoside
-
Spermine(N3BBB)
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-α-D-glucopyranose
-
DBCO-PEG6-acid
-
endo-BCN-PEG3-Maleimide
-
Ac4ManNAz
-
1-Azido-1-deoxy-β-D-galactopyranoside tetraacetate
-
2-Azidoethyl 2,3,4,6-Tetra-O-acetyl-β-D-galactopyranoside