Notes of Solid Phase Synthesis
This entry is from Wikipedia, the leading user-contributed encyclopedia.
• Combinatorial chemistry
• Protecting group
• Noncovalent solid-phase organic synthesis
Combinatorial chemistry
Combinatorial chemistry involves the rapid synthesis or the computer simulation of a large number of different but structurally related molecules.
Synthesis of molecules in a combinatorial fashion can quickly lead to large numbers of molecules. For example, a molecule with three points of diversity (R1, R2, and R3) can generate NR1 X NR2 X NR3 possible structures, where NR1, NR2 and NR3 and are the number of different substituents utilized.
Although combinatorial chemistry has only really been taken up by industry since the 1990s, its roots can be seen as far back as the 1960s when a researcher at Rockefeller University, Bruce Merrifield, started investigating the solid-phase synthesis of peptides. In the 1980s researcher H. Mario Geysen developed this technique further, creating arrays of different peptides on separate supports.
In its modern form, combinatorial chemistry has probably had its biggest impact in the pharmaceutical industry. Researchers attempting to optimize the activity profile of a compound create a ‘library’ of many different but related compounds. Advances in robotics have led to an industrial approach to combinatorial synthesis, enabling companies to routinely produce over 100,000 new and unique compounds per year.
In order to handle the vast number of structural possibilities, researchers often create a ‘virtual library’, a computational enumeration of all possible structures of a given pharmacophore with all available reactants. Such a library can consist of thousand to millions of ‘virtual’ compounds. The researcher will select a subset of the ‘virtual library’ for actual synthesis, based upon various calculations and criteria.
Materials science has applied to the techniques of combinatorial chemistry to the discovery of new materials. This work was pioneered by P.G. Schultz et al. in the mid nineties (Science, 1995, 268: 1738-1740) in the context of luminescent materials obtained by co-deposition of elements on a silicon substrate. Work has been continued by several academic groups as well as companies with large research and development programs.
External links
• Journal of Combinatorial Chemistry
• Molecular Diversity
• Combinatorial Chemistry and High Throughput Screening
• Combinatorial Chemistry: an Online Journal
• SmiLib – A free open-source software for combinatorial library enumeration
Protecting group
A Protecting group or protective group is introduced into a molecule by chemical modification of a functional group in order to obtain chemoselectivity in a subsequent chemical reaction. It plays an important role in multistep organic synthesis.
In many preparations of delicate organic compounds, some specific parts of their molecules cannot survive the required reagents or chemical environments. Then, these parts, or groups, must be protected. For example, lithium aluminum hydride is a highly reactive but useful reagent capable of reducing esters to alcohols. It will always react with carbonyl groups, and this cannot be discouraged by any means. When a reduction of an ester is required in the presence of a carbonyl, the attack of the hydride on the carbonyl has to be prevented. For example, the carbonyl is converted into an acetal, which does not react with hydrides. The acetal is then called a protecting group for the carbonyl. After the step involving the hydride is complete, the acetal is removed (by reacting it with an aqueous acid), giving back the original carbonyl. This step is called deprotection.
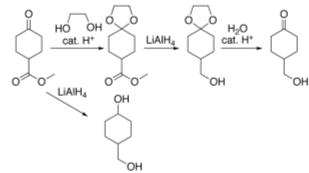
Acetal protection of a ketone during reduction of an ester
Protecting groups are more commonly used in small-scale laboratory work and initial development than in industrial production processes because their use adds additional steps and material costs to the process.
Common protecting groups
Alcohol protecting groups
• Acetyl (Ac) – Removed by acid or base.
• β-Methoxyethoxymethyl ether (MEM) – Removed by acid.
• Methoxymethyl ether (MOM) – Removed by acid.
• p-Methoxybenzyl ether (PMB) [1] – Removed by acid, hydrogenolysis, or oxidation.
• Methylthiomethyl ether – Removed by acid.
• Pivaloyl (Piv) – Removed by acid, base or reductant agents. It is stronger than other acyl protecting groups.
• Tetrahydropyran (THP) – Removed by acid.
• Silyl ether (most popular ones include trimethylsilyl (TMS), tert-butyldimethylsilyl (TBDMS), and triisopropylsilyl (TIPS) ethers) – Removed by acid or fluoride ion.
Amine protecting groups
• Carbobenzyloxy (Cbz) group – Removed by hydrogenolysis
• tert-Butyloxycarbonyl (BOC) group (Common in solid phase peptide synthesis) – Removed by concentrated, strong acid. (such as HCl or CF3COOH)
• 9-Fluorenylmethyloxycarbonyl (FMOC) group (Common in solid phase peptide synthesis) – Removed by base, such as piperidine.
• Benzyl (Bn) group – Removed by hydrogenolysis
• p-methoxyphenyl (PMP) group – Removed by Ammonium cerium(IV) nitrate (CAN)
Carbonyl protecting groups
• Acetals and Ketals – Removed by acid. Normally, the cleavage of acyclic acetals is easier than of cyclic acetals.
• Acylals – Removed by lewis acid.
• Dithianes – Removed by metal salts or oxidizing agents
Carboxylic acid protecting groups
• Methyl esters – Removed by acid or base.
• Benzyl esters – Removed by hydrogenolysis.
• tert-Butyl esters – Removed by acid, base and some reductants.
• Silyl esters – Removed by acid, base and organometallic reagents.
Noncovalent solid-phase organic synthesis
Noncovalent solid-phase organic synthesis or NC-SPOS is a form of Solid-phase synthesis where by the organic substrate is bonded to the solid phase not by a covalent bond but by other chemical interactions. This bond may consist of an induced dipole interaction between a hydrophobic matrix and a hydrophobic anchor. As long as the reaction medium is hydrophilic (polar) in nature the anchor will remain on the solid phase. Switching to a nonpolar solvent releases the organic substrate containing the anchor.
In one experimental setup [1] the hydrophobic matrix is RP silica gel (C18) and the anchor is acridone. Acridone is N-alkylated and the terminal alkene group is converted into an aldehyde by ozonolysis. This compound is bonded to RP silica gel and this system is subjected to a tandem sequence of organic reactions. The first reaction is a Barbier reaction with propargylic bromide in water (green chemistry) and the second reaction is a Sonogashira coupling. Substrates may vary in these sequences and in this way a chemical library of new compounds can be realized.
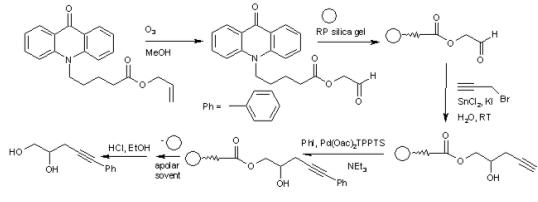
The phosphorus ligand in the Sonogashira coupling with phenyliodine is the water soluble TPPTS ligand
References
1. Porzelle, Achim; Fessner, Wolf–Dieter (2005). “Reversible Substrate Anchoring: NC-SPOS as a Sustainable Approach to Solid-Supported Organic Synthesis”. Angewandte Chemie International Edition 44 (30): 4724–4728.


