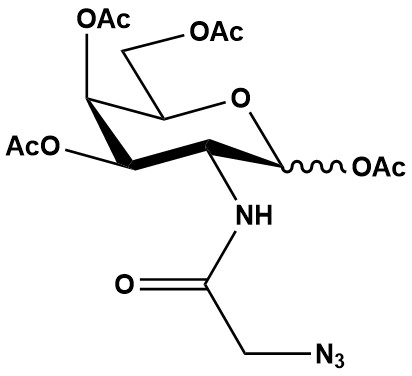
| Synonym: | N-Azidoacetylgalactosamine-tetraacylated |
| CAS #: | 653600-56-7 |
| Molecular Formula: | C16H22N4O10 |
| Molecular Weight: | 430.4 |
| Ac4GalNAz (N-Azidoacetylgalactosamine-tetraacetylated) is a widely utilized chemical tool in glycobiology and chemical biology, particularly for metabolic labeling and subsequent bioorthogonal “click chemistry” applications. Its unique properties and reactivity make it invaluable for studying glycosylation, protein trafficking, and for developing novel diagnostic and therapeutic strategies. 1. Mechanism of Action and Metabolic Labeling Ac4GalNAz functions as a “metabolic chemical reporter” (MCR). The key steps in its application are: 1.1 Cell Permeability The acetyl groups on Ac4GalNAz enhance its lipophilicity, allowing it to readily cross the cell membrane. 1.2 Intracellular Deacetylation Once inside the cell, intracellular carboxyesterases remove the acetyl groups, unmasking the free hydroxyl groups and making it resemble the natural monosaccharide N-acetylgalactosamine (GalNAc). 1.3 Metabolic Incorporation The deacetylated N-azidoacetylgalactosamine is then channeled into the cell’s natural glycosylation pathways, specifically the hexosamine biosynthetic pathway. It acts as a substrate for glycosyltransferases (e.g., polypeptide GalNAc transferases, ppGalNAcTs) and is metabolically incorporated into newly synthesized glycoconjugates, primarily mucin-type O-linked glycoproteins, which are abundant on the cell surface. This process introduces an azide tag into cellular glycans. 1.4 Bioorthogonal Ligation (Click Chemistry) The azide group is a bioorthogonal handle, meaning it reacts specifically and efficiently with complementary chemical functionalities without interfering with endogenous biological processes. The most common “click chemistry” reactions used with Ac4GalNAz are: • Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC): This reaction, while highly efficient, requires a copper(I) catalyst, which can be cytotoxic. • Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) or Copper-Free Click Chemistry: This reaction utilizes strained cyclooctynes (e.g., DBCO, BCN) that react with azides without the need for a copper catalyst, making it more suitable for live-cell and in vivo applications. • Through these reactions, a reporter molecule (e.g., fluorescent dye, biotin, affinity tag) containing an alkyne or cyclooctyne can be selectively attached to the azide-labeled glycoconjugates. 2. Applications Ac4GalNAz has revolutionized the study of glycosylation and has a broad range of applications: 2.1 Glycoprotein and Glycan Research • Visualization and Imaging: Used to fluorescently label cell surface glycoproteins, enabling their visualization by microscopy, flow cytometry, or in gel fluorescence. This allows for studies of glycoprotein trafficking, distribution, and dynamics. • Proteomic Analysis: Facilitates the enrichment and identification of glycosylated proteins. After metabolic labeling and click chemistry with a biotinylated alkyne, the labeled proteins can be isolated using streptavidin beads and subsequently identified by mass spectrometry (proteomics). • Studying Glycosyltransferase Activities: Can be used to probe the substrate specificities of various glycosyltransferases. • Analysis of Post-Translational Modifications: Particularly useful for studying O-linked β-N-acetylglucosamine (O-GlcNAc) modifications, although there can be cross-reactivity with GlcNAz. 2.2 Cell Biology and Diagnostics • Cell Surface Engineering: Allows for the modification of cell surfaces with unnatural functional groups, which can be exploited for various cellular functionalities, such as enhanced adhesion, immune evasion, or homing. • Cell Tracking: Used for in vivo cell tracking by labeling cells with an azide handle that can be detected by imaging modalities after reaction with a suitable probe. • Development of Diagnostic Tools: Plays a role in assays for detecting specific pathogens or biomarkers by leveraging its ability to label specific glycans. 2.3 Drug Discovery and Therapeutics • Targeted Drug Delivery: Facilitates the development of targeted therapies, especially in cancer treatment, by enabling the delivery of drugs to specific cells or tissues via modified glycans. • Antibody-Drug Conjugates (ADCs): Can be used in the synthesis of site-specific ADCs by introducing azide tags onto antibodies, which can then be conjugated to cytotoxic drugs via click chemistry. • PROTAC Synthesis: Ac4GalNAz can serve as a linker in the synthesis of Proteolysis-Targeting Chimeras (PROTACs), which are molecules designed to degrade target proteins. • Vaccine Development: Utilized in the design of glycoconjugate vaccines to enhance immune responses by effectively presenting carbohydrate antigens. 3. Advantages • Bioorthogonality: The azide group is largely inert to biological systems, allowing for highly specific labeling without perturbing native biochemical processes. • High Specificity: When incorporated into specific glycosylation pathways, it allows for the selective labeling of distinct glycan types (e.g., mucin-type O-linked glycans). • Versatility: Compatible with various click chemistry reactions (CuAAC, SPAAC), offering flexibility for different experimental setups (e.g., in vitro, live cell, in vivo). • Cell Permeability: The acetyl groups ensure efficient uptake by cells. • Non-Radioactive: Provides a safe and convenient alternative to traditional radioactive labeling methods. 4. Challenges and Limitations Despite its advantages, Ac4GalNAz and similar metabolic chemical reporters face certain limitations: • Metabolic Cross-talk and Specificity Issues: While generally assumed to label mucin O-linked glycans, Ac4GalNAz can be enzymatically epimerized at the 4-hydroxyl position in vivo to N-azidoacetylglucosamine (GlcNAz). This can lead to a mixture of cell-surface and O-GlcNAc labeling, complicating the interpretation of results and potentially affecting probe specificity. This epimerization is mediated by UDP-Glc/GlcNAc C4-epimerase (GALE). • Efficiency of Incorporation: The efficiency of metabolic incorporation can be influenced by the cell type, the concentration of Ac4GalNAz, and the efficiency of endogenous salvage pathways and glycosyltransferases. Metabolic bottlenecks, such as the UDP-GlcNAc pyrophosphorylase step, can limit the biosynthesis of azide-tagged nucleotide sugars, impacting labeling efficiency. • Potential for Perturbing Glycosylation: While generally considered non-toxic at optimal concentrations, higher concentrations of azidosugars could potentially interfere with normal cellular processes, including endogenous glycosylation pathways, or inhibit cellular functions (e.g., proliferation, viability). Optimization of concentration is crucial (typically 25-75 μM for cell labeling). • Toxicity of Copper Catalyst (for CuAAC): When using CuAAC, the copper(I) catalyst can be toxic to living cells, limiting its use in live-cell or in vivo applications. This necessitates the use of copper-free click chemistry (SPAAC) for such experiments. • Background Labeling: While bioorthogonal, some level of non-specific or background labeling can occur, particularly if the azide-modified sugars participate in unexpected metabolic pathways or if there is chemical reactivity with other cellular components (e.g., cysteine residues). 5. Recent Research Advancements Recent research continues to refine the use of Ac4GalNAz and similar metabolic reporters: • Improved Reporter Design: Efforts are ongoing to design new MCRs with enhanced specificity and reduced metabolic cross-talk. For example, 4-deoxy-4-fluoro-GalNAz (4FGalNAz) has been developed to be more selective for O-GlcNAc modifications by hindering epimerization. • Enhanced Labeling Efficiency: Metabolic engineering strategies, such as bypassing metabolic bottlenecks with engineered enzymes (e.g., engineered pyrophosphorylases like AGX1), have shown promise in boosting nucleotide-sugar biosynthesis and significantly increasing bioorthogonal cell surface labeling efficiency. • Novel Applications in Drug Delivery and Imaging: Ac4GalNAz is being explored further in the development of targeted drug delivery systems, including those utilizing nanoparticles and cell-based drug carriers, and for advanced in vivo imaging techniques. • Discovery of GlycoRNA: Interestingly, recent studies have shown that mammalian cells labeled with Ac4GalNAz can produce small noncoding and nonpolyadenylated glyco-modified RNA (glycoRNA), primarily localized within exosome vesicles. This finding suggests a new and unexpected role for glycosylation in nucleic acid biology and opens up new avenues for research. • Generation of “ClickECMs”: Ac4GalNAz has been used to generate azide-bearing cell-derived extracellular matrices (“clickECMs”) which combine the functional properties of natural ECM with the presence of metabolically incorporated abiotic functional groups, creating novel biomaterials. Ac4GalNAz is a versatile tool in glycobiology, enabling the metabolic labeling of glycoproteins for various applications, including imaging, proteomics, and tissue engineering. Its bioorthogonal azide functionality allows for selective conjugation with probes and therapeutics, facilitating advanced studies in cell biology and regenerative medicine. References: 1. Ac4GalNAz 2. Bioorthogonal Labeling and Chemoselective Functionalization of Lung Extracellular Matrix 3. A metabolic labeling approach for glycoproteomic analysis reveals altered glycoprotein expression upon GALNT3 knockdown in ovarian cancer cells 4. Metabolic precision labeling enables selective probing of O-linked N-acetylgalactosamine glycosylation 5. Overview of Click Chemistry |
|
Ac4GalNAz
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
Ac4GlcNAlk
-
DBCO-PEG45-acid
-
1,3,4,6-Tetra-O-acetyl-2-[(azidoacetyl)amino]-2-deoxy-β-D-galactopyranose
-
1-Azido-1-deoxy-β-D-galactopyranoside tetraacetate
-
Propargyl α-D-Mannopyranoside
-
N-Azidoacetyl-β-D-glucosamine tetraacetate
-
1,3,4,6-Tetra-O-β-acetyl-N-azidoacetylmannosamine
-
Spermine(N3HHH)•3HCl
-
N-Azidoacetyl-D-glucosamine