| CAS #: | 159857-79-1 |
| Molecular Formula: | C18H29N5O4 |
| Molecular Weight: | 379.5 |
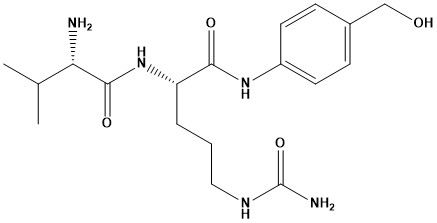
| Val-Cit-PAB (CAS number 159857-79-1), or Valine-Citrulline-p-Aminobenzylcarbamate, is a dipeptide-based linker molecule. Its primary role is to connect a therapeutic payload (a potent cytotoxic drug) to a targeting molecule, most commonly an antibody, to form an Antibody-Drug Conjugate (ADC). This linker is designed to be stable in the bloodstream but rapidly cleaved inside target cells, ensuring that the toxic drug is released precisely where it is needed, minimizing damage to healthy tissues. 1. Chemical Structure and Properties The molecule is composed of three main parts: • Valine (Val): An amino acid. • Citrulline (Cit): A non-proteinogenic amino acid. • p-Aminobenzylcarbamate (PAB): A self-immolative spacer group. The molecular formula for Val-Cit-PAB-OH is C18H29N5O4, with a molecular weight of approximately 379.46 g/mol. It is typically a white to off-white solid. The linker is often modified with other groups, such as Maleimide (MC-Val-Cit-PAB) or Fmoc (Fmoc-Val-Cit-PAB), for specific conjugation chemistry. 2. Mechanism of Action in Drug Delivery The efficacy of Val-Cit-PAB as an ADC linker is rooted in its highly selective two-step cleavage mechanism: • Targeting and Internalization: An ADC containing the Val-Cit-PAB linker binds to a specific antigen on the surface of a target cell, such as a cancer cell. The entire ADC-antigen complex is then internalized into the cell via a process called endocytosis, forming an endosome. The endosome matures into a lysosome. • Enzymatic Cleavage: Lysosomes are cellular organelles rich in hydrolytic enzymes. The Val-Cit-PAB linker is specifically designed to be a substrate for the lysosomal protease cathepsin B, which is often overexpressed in cancer cells. Cathepsin B cleaves the peptide bond between the Citrulline and PAB moieties. • Self-Immolative Release: Once cleaved from the dipeptide, the PAB group undergoes a spontaneous 1,6-elimination reaction. This rapid, non-enzymatic reaction causes the PAB spacer to break apart, cleanly releasing the active, free cytotoxic drug (the “payload”). This ensures that the drug is released in its most potent form, ready to exert its effect on the cancer cell. This mechanism ensures that the drug remains inert in the bloodstream, reducing systemic toxicity, and is only activated and released inside the target cell. 3. Applications and Research Studies Val-Cit-PAB is one of the most widely used and well-studied cleavable linkers in the field of Antibody-Drug Conjugates. It has been instrumental in the development of several FDA-approved and clinical-stage ADCs. • ADCs in Oncology: Its most significant application is in cancer therapy. Approved drugs like brentuximab vedotin (Adcetris) and polatuzumab vedotin (Polivy) use a derivative of this linker to deliver potent payloads, such as monomethyl auristatin E (MMAE), to lymphoma cells. • Pharmacokinetic Stability: A major area of research has been to improve the linker’s stability in plasma. While stable in human serum, it has been shown to be susceptible to premature cleavage by certain enzymes in some species, such as carboxylesterase 1C (Ces1C) in mice. This has led to the development of modified linkers, like those with hydrophilic P3 amino acid substitutions (e.g., Glu-Val-Cit), to enhance plasma stability and prevent off-target drug release. • Novel Therapeutic Areas: Beyond oncology, researchers are exploring the use of Val-Cit-PAB and similar cleavable linkers for other applications, including targeted delivery of therapeutics for inflammatory diseases and infectious diseases. 4. Synthesis Methods The synthesis of Val-Cit-PAB involves multi-step organic chemistry. A common approach involves coupling the protected amino acids, Valine and Citrulline, followed by a final coupling step with p-aminobenzyl alcohol. • Fmoc Solid-Phase Synthesis: One of the most common methods is solid-phase peptide synthesis (SPPS), often utilizing Fmoc (Fluorenylmethyloxycarbonyl) protecting groups. This allows for a stepwise, automated synthesis of the peptide chain. • Solution-Phase Synthesis: An alternative route is solution-phase synthesis. A documented method involves the use of coupling reagents like N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ) to facilitate the amide bond formation between the Val-Cit dipeptide and the PAB alcohol. Researchers have also explored alternative coupling agents like HATU to improve yield and reduce epimerization. Val-Cit-PAB is a well-established, effective lysosomal-cleavable linker frequently used in ADC development because it couples selective protease sensitvity (Val-Cit) with a self-immolative PAB spacer for clean payload release. However, its behavior is context-dependent — conjugation site, payload sterics, and species-specific plasma enzymes can cause premature release. When designing or interpreting ADC experiments, verify stability in the biology model you plan to use (human vs. mouse plasma), consider analytical LC–MS confirmation of released payload, and evaluate alternative linker designs if premature cleavage is observed. |
|
Val-Cit-PAB
For Research & Development use only. Not for testing and/or use on humans.