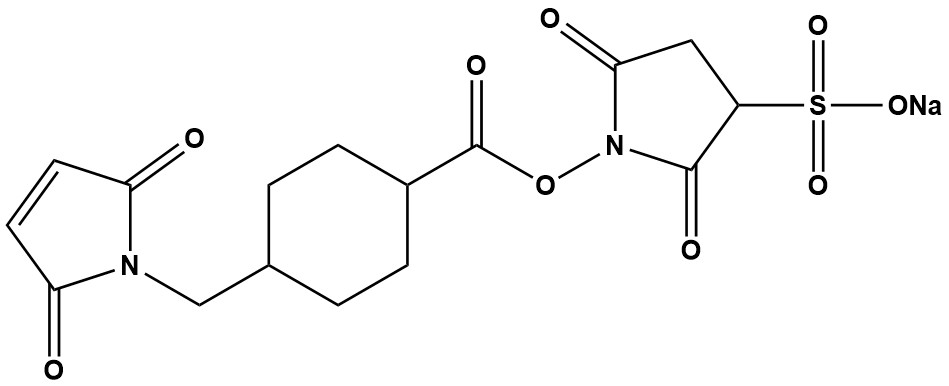
| Synonym: | Sulfo-SMCC sodium 4-(N-Maleimidomethyl)cyclohexane-1-carboxylic acid 3-sulfo-N-hydroxysuccinimide ester sodium salt |
| CAS #: | 92921-24-9 |
| Molecular Formula: | C16H17N2NaO9S |
| Molecular Weight: | 436.4 |
| Sulfo-SMCC is one of the most popular and widely used heterobifunctional crosslinking agents in biochemistry, molecular biology and bioconjugation. It belongs to the N-hydroxysuccinimide (NHS) ester and maleimide crosslinker family. Its key feature is its heterobifunctionality, meaning it possesses two different reactive groups that allow for the sequential, two-step conjugation of two different biomolecules, most commonly a protein (like an antibody) and another molecule (like a peptide, drug or enzyme).The “Sulfo-” prefix indicates the presence of a sulfonate (-SO₃⁻) group on the NHS ring, which confers water solubility on the molecule. This is a critical advantage over its parent compound, SMCC, as it allows for reactions to be performed in aqueous buffers without prior dissolution in organic solvents like DMSO, which can denature sensitive proteins. 1. Chemical Structure and Reactive Groups Sulfo-SMCC has two distinct reactive groups separated by a cyclohexane spacer: 1.1 NHS Ester (Sulfo-NHS Ester): This group reacts efficiently with primary amines (-NH₂), such as the epsilon-amino group of lysine residues or the N-terminus of proteins and peptides. The reaction forms a stable amide bond, releasing N-hydroxysulfosuccinimide. • Reaction: R-NH₂ + Sulfo-NHS ester → Stable Amide Bond + NHS byproduct 1.2 Maleimide Group: This group reacts specifically with sulfhydryl groups (-SH), such as those found on cysteine residues. The reaction forms a stable thioether bond. • Reaction: R-SH + Maleimide → Stable Thioether Bond The cyclohexane spacer provides rigidity and distance between the two conjugated molecules, which can help preserve the functionality of both by reducing steric hindrance. 2. Mechanism of Action: The Two-Step Conjugation Protocol The primary strength of Sulfo-SMCC is the ability to control the conjugation process in two distinct steps, preventing self-polymerization. Step 1: Amine Modification (Activation) The protein containing primary amines (e.g., an antibody) is first incubated with Sulfo-SMCC. The Sulfo-NHS ester group reacts with the lysine amines, covalently attaching the crosslinker and “activating” the protein with multiple maleimide groups. Excess, unreacted Sulfo-SMCC is then removed using desalting chromatography (e.g., a PD-10 column) or dialysis. Step 2: Thiol Conjugation (Coupling) The freshly “maleimide-activated” protein is then immediately mixed with the second molecule, which must contain a free thiol group (e.g., a cysteine-containing peptide, a reduced antibody fragment (Fab’), or a thiolated drug molecule). The maleimide groups on the first protein react with the thiols on the second molecule, forming a stable, covalent conjugate. This sequential process ensures a defined, 1:1 (or controlled ratio) conjugation between the two different entities. 3. Key Applications Sulfo-SMCC is a workhorse reagent with diverse applications: • Antibody-Drug Conjugates (ADCs): A classic use case. The antibody is activated with Sulfo-SMCC, and then coupled to a cytotoxic drug that has been engineered with a thiol or cysteinyl linker. • Enzyme-Protein/Peptide Conjugates: Creating complexes for immunoassays (e.g., ELISA), where an enzyme like Horseradish Peroxidase (HRP) is conjugated to an antibody or streptavidin. • Peptide-Carrier Protein Conjugation: Critical for immunology research. A small, synthetic peptide (hapten) is conjugated to a large carrier protein (e.g., KLH, BSA) using Sulfo-SMCC to generate an immune response. • Biomaterial and Surface Functionalization: Immobilizing proteins or peptides onto solid surfaces (e.g., biosensors, microarrays, nanoparticles) that have been pre-coated with amine- or thiol-reactive groups. • Oligonucleotide-Protein Conjugates: Conjugating proteins to thiol-modified DNA or RNA oligonucleotides for applications in diagnostics and nanotechnology. 4. Advantages and Strengths • Water Solubility: The sulfo-NHS group eliminates the need for organic solvents, protecting protein integrity. • Specificity: The two reactive groups target different functional groups (amines vs. thiols), allowing for controlled, directional conjugation. • Stability of Linkages: Both the amide and thioether bonds formed are highly stable under physiological conditions, leading to a durable conjugate. • Well-Established Protocol: Its use is documented in thousands of publications, making it a reliable and trusted choice. • Spacer Arm: The cyclohexane ring provides a ~11.6 Å spacer arm, which can help maintain the activity of conjugated biomolecules. 5. Limitations and Critical Considerations 5.1 Hydrolysis of Reactive Groups: • The Sulfo-NHS ester is highly susceptible to hydrolysis in aqueous solution. The reaction with amines must be performed in anhydrous, amine-free buffers (e.g., PBS, HEPES, carbonate-bicarbonate, pH 7-9) and completed quickly (within 30-60 minutes). • The Maleimide group can also undergo hydrolysis, especially at higher pH (>8.5), which renders it non-reactive toward thiols. It can also undergo a slow side reaction with amine groups if no thiols are present, though its reactivity with thiols is significantly faster at neutral pH. 5.2 pH Dependence: • NHS-ester reaction: Optimal at pH 7.2-9. Avoid buffers containing primary amines (e.g., Tris, glycine). • Maleimide reaction: Optimal at pH 6.5-7.5. The thiol group must be in its deprotonated (S⁻) form for efficient reaction, which occurs above its pKa (~8.6 for cysteine). However, maleimide reactivity is specific enough to work well at pH 7.0, where hydrolysis is minimized. 5.3 Thiol Source: • The target molecule must possess a free thiol. This often requires introducing a cysteine residue via genetic engineering or chemically reducing disulfide bonds (e.g., with TCEP or DTT), with careful removal of the reducing agent before conjugation. 6. Handling, Storage, and Stability • Storage: Sulfo-SMCC is typically supplied as a white powder. It must be stored desiccated at -20°C or -80°C and protected from moisture. Under these conditions, it is stable for years. • Reconstitution: The entire vial should be warmed to room temperature before opening to prevent condensation and hydrolysis. It should be dissolved immediately in a cold, anhydrous buffer just before use. • Solution Stability: Once in aqueous solution, it begins to degrade rapidly. The activated protein from Step 1 should be used immediately for the best results. Sulfo-SMCC remains a gold-standard heterobifunctional crosslinker for creating defined covalent conjugates between amine-containing and thiol-containing molecules. Its water solubility, specificity, and the stability of the bonds it forms make it an indispensable tool in modern bioconjugation. While users must be mindful of its hydrolysis sensitivity and strictly control reaction conditions, its well-optimized protocols and proven track record across countless applications cement its status as a fundamental reagent in the life science research toolkit. For any project requiring the linkage of a protein to another bioactive molecule, Sulfo-SMCC should be a primary candidate for the conjugation chemistry. |
|
Sulfo-SMCC
For Research & Development use only. Not for testing and/or use on humans.