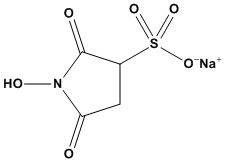
| Synonym: | N-hydroxysulfosuccinimide |
| CAS #: | 106627-54-7 |
| Molecular Formula: | C4H4NNaO6S |
| Molecular Weight: | 217.1 |
| Sulfo-NHS (CAS #: 106627-54-7), also known as Sulfo-N-Hydroxysuccinimide, is a highly water-soluble, next-generation activation reagent widely used to enhance EDC-mediated carboxyl-to-amine coupling in bioconjugation and surface chemistry. Featuring a sulfonated succinimide ring, Sulfo-NHS efficiently converts unstable O-acylisourea intermediates into stable Sulfo-NHS esters, delivering superior reaction yields, cleaner product profiles, and improved reproducibility in aqueous environments. Designed specifically for biochemical and biomaterials applications, Sulfo-NHS enables reliable modification of proteins, peptides, antibodies, enzymes, nanoparticles, hydrogels, and carboxyl-functional surfaces. Its exceptional water solubility eliminates the need for organic solvents like DMSO or DMF, making it ideal for sensitive biological systems and in-solution reactions. 1. Mechanism of Action: The Two-Step Process Sulfo-NHS is not a crosslinker on its own. It is an additive used in two-step EDC coupling to improve efficiency and control. The overall goal is to form a stable amide bond between a molecule with a carboxylic acid (R-COOH) and a molecule with a primary amine (R’-NH₂). Step 1: Carboxyl Activation (Acidic pH) This first step is typically performed in a slightly acidic buffer, such as MES buffer (pH 4.7–6.0) • A carboxylic acid (e.g., on a protein’s C-terminus, or on an aspartate/glutamate side chain) is mixed with EDC (1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide). • EDC reacts with the carboxyl group to form a highly unstable O-acylisourea intermediate. • This is where Sulfo-NHS acts. It immediately reacts with this unstable intermediate, displacing the EDC group. • The result is a Sulfo-NHS ester. This new intermediate is significantly more stable than the O-acylisourea, particularly in aqueous solutions, although it is still susceptible to hydrolysis. Step 2: Amine Conjugation (Neutral to Basic pH) This second step is most efficient at a more physiological pH. The reaction buffer is often adjusted to PBS or HEPES (pH 7.2–8.0). • The molecule containing the primary amine (e.g., a protein’s lysine side chain, an N-terminus, or an amine-modified oligonucleotide) is introduced. • The amine’s nucleophilic nitrogen attacks the carbonyl carbon of the Sulfo-NHS ester. • This attack forms a stable, covalent amide bond, permanently linking the two molecules. • The Sulfo-NHS molecule is released as a soluble, non-reactive by-product. 2. Key Advantages of Sulfo-NHS (vs. standard NHS) The primary benefit of Sulfo-NHS comes from its sulfonate group, which imparts properties that its non-sulfonated analog, NHS (N-hydroxysuccinimide), lacks. • High Water Solubility: This is the most significant advantage. Sulfo-NHS dissolves directly into aqueous buffers. Standard NHS is hydrophobic and must first be dissolved in an organic solvent (like DMSO or DMF) and then added to the reaction, which can introduce solvents that denature or precipitate sensitive proteins. • Membrane Impermeability: The negative charge on the sulfonate group prevents Sulfo-NHS from passively crossing intact cell membranes. This makes it the reagent of choice for cell surface labeling, as it will only activate carboxyls on the exterior of the cell, leaving intracellular proteins untouched. Standard NHS can diffuse across the membrane. • Increased Reaction Efficiency: By “capturing” the unstable EDC intermediate and converting it into a semi-stable ester (half-life of hours at pH 7 vs. minutes for the EDC intermediate), Sulfo-NHS dramatically reduces the competing hydrolysis reaction. This leads to a higher overall yield of the final desired conjugate. 3. Common Applications in Research Sulfo-NHS is a workhorse reagent in biochemistry and molecular biology for: • Protein and Antibody Labeling: Activating carboxyl groups on a protein or antibody so it can be conjugated to an amine-containing label, such as a fluorescent dye (e.g., FITC-amine), biotin-amine, or an enzyme (e.g., HRP). • Immobilization onto Surfaces: Activating carboxyl-coated surfaces (like sensor chips for SPR/Biacore, ELISA plates, or magnetic beads) so that proteins, peptides, or other amine-ligands can be covalently and stably attached. • Cell Surface Protein Studies: Selectively labeling or modifying proteins on the outer leaflet of a cell’s plasma membrane, without affecting the cell’s interior. • Creation of Bioconjugates: Forming stable crosslinks between two different proteins (one with a -COOH, one with an -NH₂), or between a protein and a peptide, or a protein and a drug molecule. 4. Protocol Considerations and Limitations While powerful, using Sulfo-NHS successfully requires attention to detail. 4.1 Hydrolysis is the Enemy • The primary competing reaction is the hydrolysis (breakdown by water) of the Sulfo-NHS ester. This reaction is highly pH-dependent and increases dramatically at higher pH. This is why the conjugation step (Step 2) is a race between the desired amine reaction and the undesired hydrolysis. 4.2 Buffer Choice is Critical • Do NOT use buffers that contain primary amines, such as Tris (Tris(hydroxymethyl)aminomethane) or glycine. These amines will compete with your target molecule and quench the reaction. • Good Buffers (Step 1): MES is the most common choice, as it buffers well in the acidic pH range (4.7-6.0) and lacks amines. • Good Buffers (Step 2): PBS (Phosphate-Buffered Saline) or HEPES are ideal, as they buffer well at the neutral/basic pH range (7.2-8.0) and are amine-free. 4.3 Reagent Stability and Storage • Powder: Sulfo-NHS powder is hygroscopic (absorbs moisture from the air). This moisture will hydrolyze the reagent, rendering it inactive. It must be stored at -20°C in a desiccator. • In Solution: Do not pre-make stock solutions. Sulfo-NHS hydrolyzes in water. It should be weighed out and dissolved in the reaction buffer immediately before use. 4.4 Quenching the Reaction • After the desired reaction time, any remaining active Sulfo-NHS esters can be “quenched” (deactivated) by adding an excess of a primary amine, such as Tris, glycine, or hydroxylamine. Sulfo-NHS is an indispensable tool for bioconjugation. By adding water solubility and membrane-impermeability to the standard EDC/NHS coupling chemistry, it enables more efficient, gentle and specific modification of proteins and surfaces in aqueous environments. |
|
Sulfo-NHS
For Research & Development use only. Not for testing and/or use on humans.