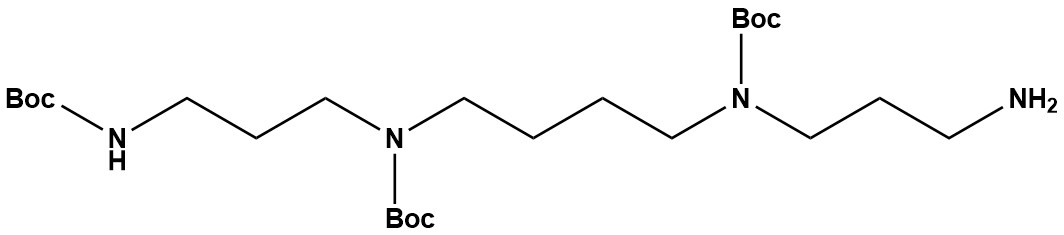
| Synonym: | Tris-Boc-spermine; N1,N4,N9-tri-Boc-spermine |
| CAS #: | 114459-62-0 |
| Molecular Formula: | C25H50N4O6 |
| Molecular Weight: | 502.7 |
| Spermine(HBBB) is a synthetically protected form of spermine, a naturally occurring tetraamine involved in numerous cellular processes. In this modified structure, spermine’s reactive amine groups are protected by tert-butoxycarbonyl (Boc) to control reactivity during synthetic steps.These protections make it particularly suitable for solid-phase peptide synthesis (SPPS) and conjugation chemistry, especially in designing peptidomimetics, nucleic acid delivery systems, and bioactive ligands. 1. Chemical Structure and Properties • Core Structure: The underlying molecule is spermine (N1,N4-Bis(3-aminopropyl)butane-1,4-diamine), a naturally occurring polyamine. Spermine has four amine groups: two primary amines at the ends of the chains and two secondary amines within the backbone. • Protecting Groups: “Tris-Boc” indicates that three of these four amine groups are protected by tert-butyloxycarbonyl (Boc) groups. The Boc group is a widely used protecting group for amines in organic synthesis because it is stable to a variety of reaction conditions but can be readily removed under mild acidic conditions (e.g., with trifluoroacetic acid, TFA). • Specific Protection Pattern: The “HBBB” nomenclature often suggests a specific pattern of protection, where three amines are Boc-protected, leaving one amine (likely a primary one) free. This selective protection is crucial for enabling controlled, stepwise synthesis. • Solubility: Expected to be soluble in common organic solvents (e.g., DCM, chloroform, ethyl acetate, THF) due to the presence of the lipophilic Boc groups. • Stability: Generally stable under normal storage conditions. Storage at 0-8 °C is often recommended to maintain purity. 2. Applications Spermine(HBBB) / Tris-Boc-spermine is primarily used as a versatile building block and intermediate in synthetic organic chemistry, particularly for the creation of more complex molecules that require a spermine backbone with differential reactivity. 2.1 Drug Development and Medicinal Chemistry • Polyamine Analogs: It serves as a crucial precursor for synthesizing polyamine analogs. These analogs are explored as potential therapeutic agents, especially in cancer therapy, where polyamine metabolism is often dysregulated. These derivatives can modulate polyamine pathways, act as enzyme inhibitors, or serve as carriers for cytotoxic agents. • Gene Delivery Systems: Polyamines, due to their polycationic nature, can interact with negatively charged nucleic acids (DNA, RNA). Tris-Boc-spermine can be incorporated into gene delivery vectors (e.g., cationic lipids, polymers) to improve the encapsulation and transfection efficiency of nucleic acids into cells for gene therapy applications. The Boc groups can be deprotected later to restore the polycationic character. • Antimicrobial Agents: Some spermine derivatives show antibacterial properties. Tris-Boc-spermine itself has been investigated as an antibacterial agent, particularly against Gram-positive bacteria, potentially by disrupting bacterial cell wall synthesis. • Targeted Therapies: The free amine can be selectively functionalized with targeting ligands (e.g., peptides, antibodies, carbohydrates) to create drug conjugates that deliver therapeutic payloads specifically to diseased cells. 2.2 Polymer Chemistry • Cross-linking Agent: Its specific protection pattern allows it to be used as a cross-linking agent in the formulation of biodegradable polymers. These polymers find applications in drug delivery systems, tissue engineering scaffolds, and other biomedical materials. 2.3 Molecular Probes and Diagnostic Tools • Fluorescent Probes: Tris-Boc-spermine has been described as a fluorescent probe for detecting specific ions (e.g., selenide ions). • Biosensors: Its ability to interact with biomolecules makes it useful in the development of biosensors and diagnostic assays for disease detection. 2.4 Organic Synthesis • Versatile Building Block: The combination of a multi-amine scaffold with differential protection allows it to act as a versatile building block for synthesizing a wide range of organic molecules, where controlled functionalization of multiple amine groups is required. • Linkers and Modifiers: It can be used as a linker in various applications, connecting different molecular entities, or as a modifier to introduce polyamine characteristics into a molecule. 3. Advantages • Selective Functionalization: The primary advantage is the ability to selectively deprotect the free amine (if present) or another amine (if the Boc groups are removed selectively in subsequent steps) to perform controlled coupling reactions. This allows for the synthesis of asymmetric spermine derivatives. • Boc Protection Benefits: Boc groups offer robustness during synthesis and relatively mild acid-labile deprotection, making them compatible with many synthetic strategies. • Precursor to Biologically Active Molecules: Provides a scaffold for building compounds with diverse biological activities due to the inherent properties of polyamines. 4. Challenges and Considerations • Synthetic Difficulty: The synthesis of specifically protected polyamines like Tris-Boc-spermine can be challenging due to the presence of multiple, often similarly reactive, amine groups, requiring careful control of reaction conditions and stoichiometry. • Purification: Achieving high purity is critical, as side products from partial or over-protection can be difficult to separate. • Deprotection: While Boc deprotection is generally mild, the strong acidic conditions required (e.g., neat TFA) must be compatible with any other functional groups or sensitive biomolecules present in the final conjugate. • Biological Activity of Derivatives: While spermine itself has biological roles, the biological activity of its protected or modified derivatives needs to be rigorously evaluated. Spermine(HBBB), or Tris-Boc-spermine, is a key synthetic intermediate that leverages the fascinating biological properties of polyamines while providing the necessary chemical control for precise molecular engineering. Its distinct advantage lies in its selective amine protection, enabling the rational design and synthesis of complex polyamine-based conjugates. This makes it an invaluable tool in medicinal chemistry for drug discovery (especially in oncology and gene delivery), polymer science, and the development of advanced molecular probes, opening avenues for novel therapeutic and diagnostic solutions. References 1. Spermine 2. Spermine(HBBB) |
|
Spermine(HBBB)
For Research & Development use only. Not for testing and/or use on humans.