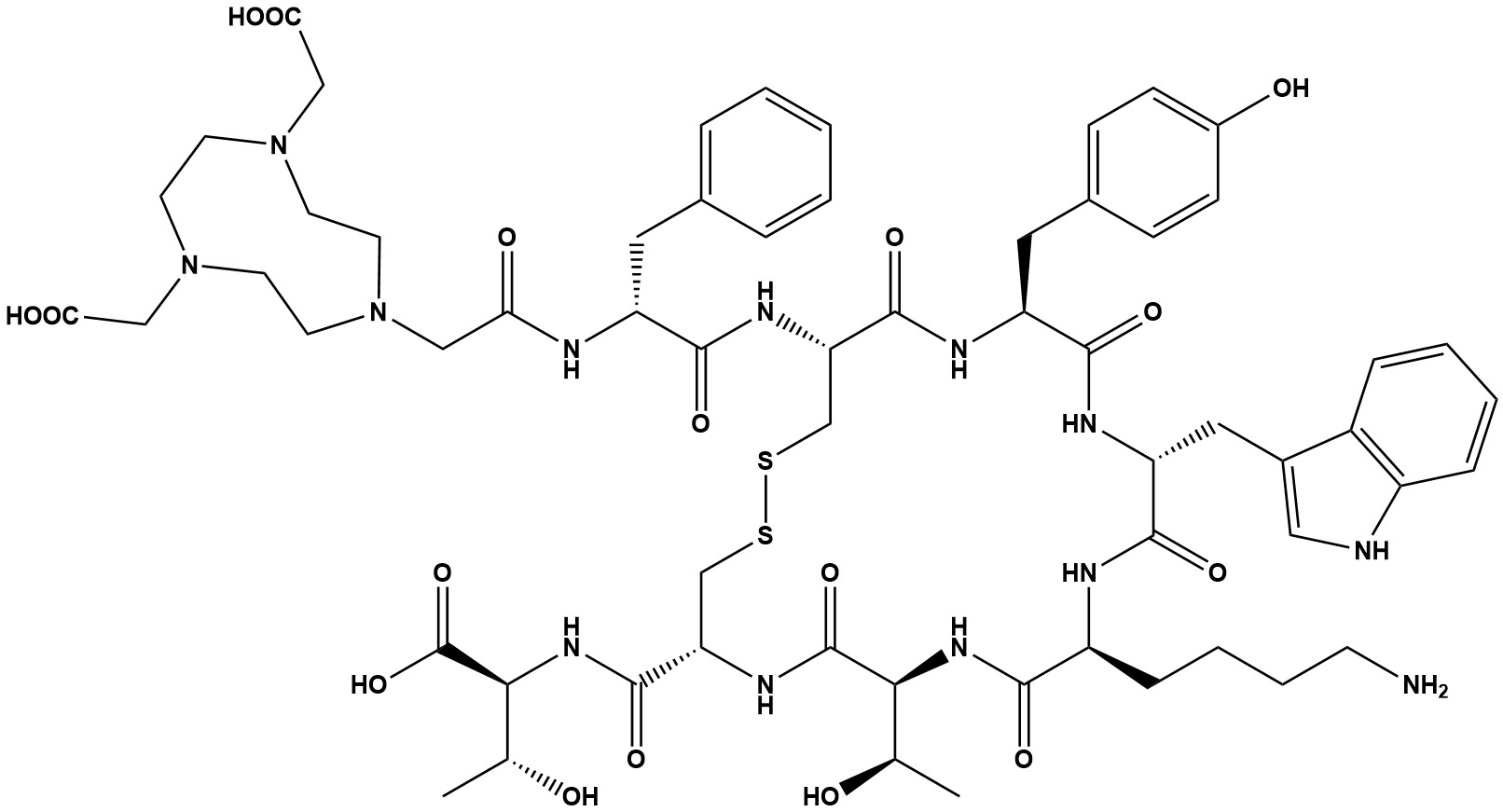
| Synonym: | NOTA-D-Phe-Cys-Tyr-D-Trp-Lys-Thr-Cys-Thr (Disulfide bridge:Cys2-Cys7) |
| CAS #: | 709043-21-0 (net) |
| Molecular Formula: | C61H83N13O17S2 |
| Molecular Weight: | 1334.5 |
| NOTA-TATE is an advanced radiopharmaceutical primarily used in the field of nuclear medicine for the diagnosis and potential treatment of neuroendocrine tumors (NETs). It is part of a class of drugs known as somatostatin receptor (SSTR) agonists. The core components of NOTA-TATE are the peptide octreotate (TATE), which targets somatostatin receptors that are overexpressed on the surface of NET cells, and the chelator NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid). 1. Radiochemical Properties and Advantages The choice of chelator, NOTA, is a key differentiating factor. NOTA is a smaller molecule with a different coordination chemistry compared to DOTA, which is used in DOTA-TATE. This difference allows NOTA-TATE to be labeled with a variety of radionuclides, most notably Fluorine-18 (18F), which is not easily achieved with DOTA. The ability to use 18F is a significant advantage for several reasons: • Logistical Benefits: 18F has a longer half−life (approximately 110 minutes) compared to Gallium−68(68Ga) (approximately 68 minutes), the radionuclide typically used with DOTA-TATE for PET imaging. This longer half-life allows for more flexible production and distribution, making it possible to produce the radiopharmaceutical at a centralized cyclotron facility and transport it to a greater number of hospitals. • Improved Image Quality: The lower positron energy of 18F results in better spatial resolution and image quality for PET/CT scans compared to 68Ga, which can potentially lead to the detection of smaller lesions. • Production: 18F is produced in a cyclotron, which can be done on a large scale, addressing some of the supply and cost limitations associated with generator-produced radionuclides like 68Ga. 2. Diagnostic Applications: 18F-NOTA-TATE PET/CT Clinical studies have shown that 18F-NOTA-TATE is a highly effective diagnostic tool for imaging SSTR-positive neuroendocrine tumors. Head-to-head comparisons with the current “gold standard,” 68Ga-DOTA-TATE, have yielded promising results: • Comparable Efficacy: Studies have demonstrated that 18F-NOTA-TATE provides comparable diagnostic accuracy to 68Ga-DOTA-TATE for detecting and staging NETs. • Improved Lesion Detection: In some clinical trials, 18F-NOTA-TATE was able to detect a greater number of lesions, particularly small lesions in the liver and lymph nodes, which is a crucial advantage for accurate staging and treatment planning. • Favorable Biodistribution: 18F-NOTA-TATE has shown lower physiological uptake in certain organs, such as the liver, salivary glands, and pancreas, compared to 68Ga-DOTA-TATE. This can result in a higher tumor-to-background ratio, leading to clearer and more confident tumor visualization. 3. Therapeutic Potential: 177Lu-NOTA-TATE While DOTA-TATE is the established standard for peptide receptor radionuclide therapy (PRRT) with Lutetium-177 (177Lu), research is ongoing into the therapeutic potential of 177Lu-NOTA-TATE. Early studies have indicated that 177Lu-NOTA-TATE has favorable properties for therapy: • Favorable Excretion Profile: It has been observed that 177Lu-NOTA-TATE is excreted more rapidly by the kidneys than its DOTA-TATE counterpart. This could potentially reduce the radiation dose to the kidneys, which is a major dose-limiting organ in PRRT. • Comparable Biological Efficacy: The TATE peptide remains the active targeting component, suggesting that 177Lu-NOTA-TATE would have similar therapeutic efficacy by delivering a localized dose of beta radiation to the tumor cells. NOTA-TATE, particularly when labeled with 18F, represents a highly promising and viable alternative to the established DOTA-TATE for the diagnosis and management of neuroendocrine tumors. Its logistical advantages, including a longer half-life and large-scale production capabilities, make it an attractive option for wider clinical availability. The superior image quality and favorable biodistribution observed in initial studies provide a strong argument for its use as a primary diagnostic agent. While 177Lu-DOTA-TATE remains the approved standard for PRRT, the potential for reduced renal toxicity with 177Lu-NOTA-TATE is an exciting area of ongoing research that could further solidify NOTA-TATE’s role in the theranostic paradigm. As more clinical data becomes available, NOTA-TATE is expected to become an increasingly important tool in the fight against neuroendocrine tumors. References: 1. A prospective head-to-head comparison of 68 Ga-NOTA-3P-TATE-RGD and 68 Ga-DOTATATE in patients with gastroenteropancreatic neuroendocrine tumours 2. Comparative evaluation of 68Ga-labelled TATEs: the impact of chelators on imaging 3. Evaluation of 18F-AlF-NOTA-octreotide for imaging neuroendocrine neoplasms: comparison with 68Ga-DOTATATE PET/CT |
|
NOTA-TATE
For Research & Development use only. Not for testing and/or use on humans.