| Synonym: | L-Glucose |
| CAS #: | 921-60-8 |
| Molecular Formula: | C6H12O6 |
| Molecular Weight: | 180.2 |
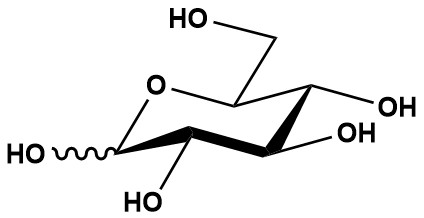
| L-Glucose, also known as L-(−)-Glucose, Levoglucose, or L-Glucopyranose, is a monosaccharide and the enantiomer of the naturally abundant D-Glucose. With the chemical formula C6H12O6 and a molecular weight of 180.16 g/mol, L-Glucose is a stereoisomer characterized by its mirror-image configuration relative to D-Glucose. Its CAS number is 921-60-8, and it is identified by the IUPAC name (2S,3R,4S,5S)-2,3,4,5,6-pentahydroxyhexanal. Unlike D-Glucose, which is a primary energy source in biological systems, L-Glucose is not metabolized by most cells due to its inability to be phosphorylated by hexokinase, a key enzyme in glycolysis. 1. Chemical and Physical Properties L-Glucose is a six-carbon aldose sugar with a linear structure containing an aldehyde group and five hydroxyl groups. Its stereochemical configuration is the mirror image of D-Glucose, with all chiral centers inverted. Key physical properties include:• Molecular Formula: C6H12O6 • Molecular Weight: 180.16 g/mol • Solubility: Soluble in water (50 mg/ml) and slightly soluble in alcohol • Appearance: Typically a white crystalline powder L-Glucose shares the same molecular formula and connectivity as D-Glucose but differs in its optical rotation, exhibiting a negative rotation (hence the designation L-(−)-Glucose). It is stable at room temperature and can be stored at +4°C for analytical purposes. 2. Biological Significance 2.1 Non-Metabolizable Nature L-Glucose is notable for its inability to serve as an energy source in most organisms. This is primarily due to its stereochemical incompatibility with hexokinase, the enzyme that phosphorylates glucose to glucose-6-phosphate in the first step of glycolysis. The active site of hexokinase is highly specific to the D-glucose configuration, preventing L-Glucose phosphorylation. However, certain bacteria, such as Klebsiella pneumoniae and Paracoccus species 43P, possess NAD+-dependent L-glucose dehydrogenases that can oxidize L-Glucose, enabling its catabolism in specific pathways. 2.2 Metabolic Studies L-Glucose is widely used as a control in metabolic studies due to its structural similarity to D-Glucose but lack of metabolic activity in most systems. For example, it has been employed to study the substrate specificity of glucose transporters, such as the IICB glucose transporter, and to investigate non-mediated glucose absorption in organisms like house sparrows (Passer domesticus). Its use in these studies helps differentiate between active transport and passive diffusion mechanisms. 2.3 Effects on Cellular Processes Research indicates that L-Glucose can influence specific cellular processes despite its non-metabolizable nature. For instance, studies have shown that L-Glucose does not suppress food intake when ingested chronically, unlike some artificial sweeteners, suggesting it does not trigger the same satiety signals as D-Glucose. Additionally, L-Glucose has been used to explore brain functions, such as memory and learning, potentially due to its ability to interact with neuronal signaling pathways. However, some sources incorrectly attribute energy-source properties to L-Glucose, likely confusing it with D-Glucose. 3. Applications 3.1 Research and Diagnostics L-Glucose is a valuable tool in biochemical and physiological research: • Metabolic Studies: Used as a non-metabolizable control to study glucose metabolism, transport, and enzymatic specificity. • Diabetes Research: Employed in model systems to mimic glucose without inducing metabolic responses, aiding in the study of diabetes-related cellular effects. • Cancer Cell Visualization: Fluorescent derivatives like 2-NBDLG (a tagged L-Glucose analog) are used to visualize cancer cells, as they are taken up in a phloretin-sensitive manner by insulinoma cells. • Memory and Learning: Investigated for potential effects on cognitive functions due to its interaction with neuronal signaling. 3.2 Pharmaceutical and Clinical Applications • Colon Cleansing Agent: L-Glucose has been explored as a colon-cleansing agent for colonoscopy preparation due to its non-absorption in the gastrointestinal tract, providing an osmotic effect without metabolic interference. • Therapeutic Potential: L-Glucose pentaacetate, a derivative, has been studied as a potential therapeutic agent for type II diabetes, though specific mechanisms remain under investigation. 3.3 Industrial Applications L-Glucose is used in the synthesis of various compounds, including L-Glucose pentaacetate and L-glucuronic acid, which have applications in pharmaceutical and chemical industries. Its role as a chiral building block is also significant in organic synthesis, particularly for creating stereospecific molecules. 4. Synthesis and Production L-Glucose is not naturally abundant and is typically synthesized chemically or through bioconversion processes. A notable bioconversion method involves the transformation of L-fructose to L-Glucose by Klebsiella pneumoniae, as described by Leang et al. (2003). Chemical synthesis often involves stereospecific reactions to produce the L-enantiomer from D-glucose or other precursors, such as the method reported by Martínez et al. (2014) for converting D-Glucose to L-Glucose and L-glucuronic acid. 5. Research Insights • Enzymatic Specificity: The inability of L-Glucose to be phosphorylated by hexokinase has been extensively studied, with research highlighting its stereochemical limitations. This property makes it an ideal negative control in glucose metabolism studies. • Bacterial Utilization: The discovery of L-Glucose catabolic pathways in bacteria like Paracoccus species 43P has opened new avenues for understanding microbial metabolism. • Neuronal Effects: Studies, such as Talley et al. (2002), suggest L-Glucose may influence spontaneous alternation performance in rats, potentially via non-metabolic interactions with neural pathways. 6. Future Directions Future research on L-Glucose could focus on: • Therapeutic Applications: Further exploration of L-Glucose derivatives in diabetes treatment and colon cleansing. • Microbial Pathways: Expanding knowledge of L-Glucose metabolism in bacteria for biotechnological applications. • Neuronal Signaling: Investigating the mechanisms by which L-Glucose affects cognitive functions. • Synthetic Methods: Developing more efficient and cost-effective synthesis routes for large-scale production. L-Glucose (CAS 921-60-8) is a fascinating enantiomer of D-Glucose with significant applications in research, diagnostics, and potential therapeutics. Its non-metabolizable nature makes it a critical tool for studying glucose metabolism, enzymatic specificity, and cellular processes. While primarily used in research, its derivatives show promise in clinical applications, and its role in microbial metabolism opens new biotechnological possibilities. As research progresses, L-Glucose is likely to remain a valuable compound in advancing biochemical and medical sciences. References: 1. L-Glucose 2. L-Glucose: Another Path to Cancer Cells 3. What is difference between D glucose and L glucose? 4. D and L Sugars |
|
L-(-)-Glucose
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
Ethyl Vanillin Glucoside
-
4-Methylphenyl 2,3,4,6-Tetra-O-acetyl-1-thio-β-D-glucopyranoside
-
4-Methylphenyl 1-Thio-β-D-glucopyranoside
-
Allyl 2,3,4,6-Tetra-O-acetyl-β-D-glucopyranoside
-
Methyl 4,6-O-Benzylidene-α-D-glucopyranoside
-
Tri-O-acetyl-D-glucal
-
Methyl β-D-Glucopyranoside
-
Methyl 2,3,4-Tri-O-benzyl-α-D-glucopyranoside
-
Ethyl β-D-Glucopyranoside