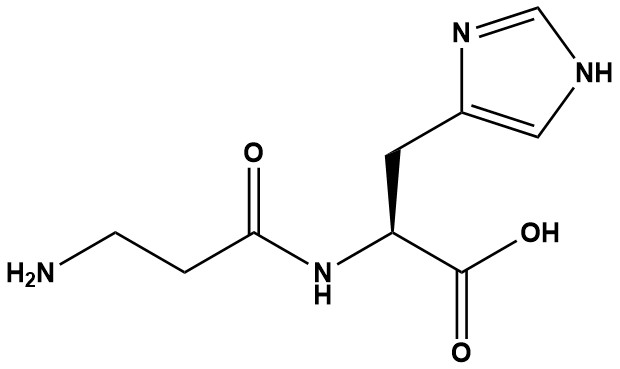
| Synonym: | Beta-Alanyl-L-Histidine; Ignotine |
| Sequence: | beta-Ala-His |
| CAS #: | 305-84-0 |
| Molecular Formula: | C9H14N4O3 |
| Molecular Weight: | 226.2 |
| Carnosine (β-alanyl-L-histidine) is a naturally occurring dipeptide composed of the amino acids beta-alanine and L-histidine. First discovered in 1900 by Russian chemist Vladimir Gulevich, carnosine is found in significant concentrations in muscle tissue, cardiac tissue, and the brain. It represents one of the most abundant non-protein nitrogenous compounds in mammalian tissues, with particularly high concentrations in skeletal muscle. 1. Chemical Properties Carnosine is a zwitterionic dipeptide with a beta-alanine residue linked to L-histidine via a peptide bond. Its imidazole ring (from histidine) confers unique chemical properties, including pH buffering (pKa ~6.83) and metal chelation (e.g., copper, zinc). Carnosine is water-soluble (logP < 0) with limited lipophilicity. It is stable under neutral to slightly acidic conditions (pH 4-7), but hydrolyzes in strong acids or bases, breaking into its constituent amino acids. 2. Mechanism of Action Carnosine’s bioactivity stems from its multifaceted roles at the cellular level: 2.1 Antioxidant • Carnosine acts as a free radical scavenger, neutralizing reactive oxygen species (ROS) and reactive nitrogen species (RNS). It also chelates divalent metal ions (e.g., copper and zinc), preventing oxidative damage via Fenton reactions. 2.2 Anti-Glycation • Carnosine inhibits the formation of advanced glycation end-products (AGEs). 2.3 pH Buffering • Carnosine is a potent intracellular pH buffer, particularly in skeletal muscle, where it helps neutralize lactic acid accumulation during high-intensity exercise. Its pKa of approximately 6.83 makes 2.4 Metal Chelation • Carnosine binds to and detoxifies excess metals such as copper and zinc, preventing metal-induced oxidative stress. 2.5 Anti-Aging • By inhibiting AGE formation and protecting against protein carbonylation, carnosine may slow cellular senescence and extend tissue lifespan. 2.6 Neuroprotection • By reducing excitotoxicity and inflammation, carnosine protects neurons from ischemic damage and amyloid-beta aggregation, relevant to Alzheimer’s and stroke. Unlike peptides like GHK-Cu, which focus on collagen synthesis, carnosine’s strength lies in systemic protection and homeostasis, making it a broad-spectrum bioactive. 3. Comparison with Alternatives • vs. GHK-Cu: Carnosine excels in antioxidation and buffering, while GHK-Cu focuses on collagen and regeneration. • vs. Glutathione: Both are antioxidants, but carnosine’s anti-glycation and buffering roles give it broader applications; glutathione is more potent against systemic ROS. • vs. Retinoids: Retinoids outperform in cell turnover and pigmentation but irritate skin; carnosine is gentler with protective benefits. Carnosine represents a multifunctional dipeptide with remarkable biological versatility. The compound’s multiple mechanisms of action – including antioxidant capacity, anti-glycation properties, metal chelation, and pH buffering – make it particularly interesting for conditions involving oxidative stress, protein damage, and metabolic dysregulation. References 1. Carnosine 2. Carnosine – an overview 3. Carnosine: A Versatile Antioxidant and Antiglycating Agent 4. l-Carnosine reduces telomere damage and shortening rate in cultured normal fibroblasts 5. The buffering of muscle in rigor; protein, phosphate and carnosine 6. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans 7. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): an overview of their mechanisms of formation 8. Carnosine: A Versatile Antioxidant and Antiglycating Agent 9. Carnosine and its constituents inhibit glycation of low-density lipoproteins that promotes foam cell formation in vitro 10. Carnosine As a Natural Antioxidant and Geroprotector: From Molecular Mechanisms to Clinical Trials 11. Understanding the behaviour of carnosine in aqueous solution: an experimental and quantum-based computational investigation on acid–base properties and complexation mechanisms with Ca2+ and Mg2+ 12. Influence of β-alanine supplementation on skeletal muscle carnosine concentrations and high intensity cycling capacity |
|
L-Carnosine
For Research & Development use only. Not for testing and/or use on humans.