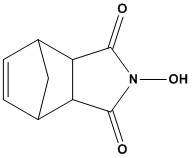
| Synonym: | N-Hydroxy-5-norbornene-2,3-dicarboximide |
| CAS #: | 21715-90-2 |
| Molecular Formula: | C9H9NO3 |
| Molecular Weight: | 179.2 |
| N-Hydroxy-5-norbornene-2,3-dicarboximide (HONB), with the CAS Number 21715-90-2, is a specialized biochemical reagent widely utilized in proteomics research, particularly in peptide synthesis. HONB is recognized for its role in reducing racemization during peptide synthesis and inhibiting the formation of N-acylureas, making it a valuable tool in the synthesis of high-purity peptides. 1. Applications in Peptide Synthesis HONB is primarily employed as a coupling reagent in solution-phase peptide synthesis. Its key applications include: 1.1 Reduction of Racemization Racemization, the unwanted conversion of chiral amino acids into their enantiomers, is a significant challenge in peptide synthesis. HONB, when used in conjunction with dicyclohexylcarbodiimide (DCC), significantly reduces racemization compared to other coupling reagents like N-hydroxysuccinimide (HOSu or NHS). This property is critical for synthesizing peptides with precise stereochemistry, such as enkephalin analogs. 1.2 Inhibition of N-Acylurea Formation During peptide coupling, DCC can form N-acylureas as byproducts, which reduce yield and complicate purification. HONB inhibits this side reaction, leading to higher yields of the desired peptide product. 1.3 Synthesis of Enkephalin Analogs HONB has been reported to be particularly effective in the synthesis of enkephalin analogs. Its superior performance over HOSu in these applications is attributed to its ability to enhance coupling efficiency and minimize side reactions. 1.4 Introduction of Amino-Protecting Groups The corresponding chloroformate of HONB can be used to introduce various amino-protecting groups, which are essential for controlling the reactivity of amino acids during peptide synthesis. 2. Advantages of HONB HONB offers several advantages over other coupling reagents: • Enhanced Coupling Efficiency: HONB’s reactivity with DCC results in more efficient peptide bond formation compared to HOSu, leading to higher yields. • Stereochemical Control: Its ability to minimize racemization ensures the production of stereochemically pure peptides, which is critical for biological activity. • Versatility: HONB is compatible with a wide range of amino acids and protecting groups, making it suitable for diverse peptide synthesis protocols. HONB (CAS 21715-90-2) is a highly effective coupling reagent in peptide and amide bond chemistry. Its compatibility with carbodiimide reagents, low racemization rates, and improved safety profile make it a versatile and essential tool in modern synthetic organic and peptide chemistry. References 1. N-hydroxy-5-norbornene-2,3-dicarboximide 2. Peptide Synthesis 3. Fmoc Solid Phase Peptide Synthesis 4. Boc Solid Phase Peptide Synthesis |
|
HONB
For Research & Development use only. Not for testing and/or use on humans.