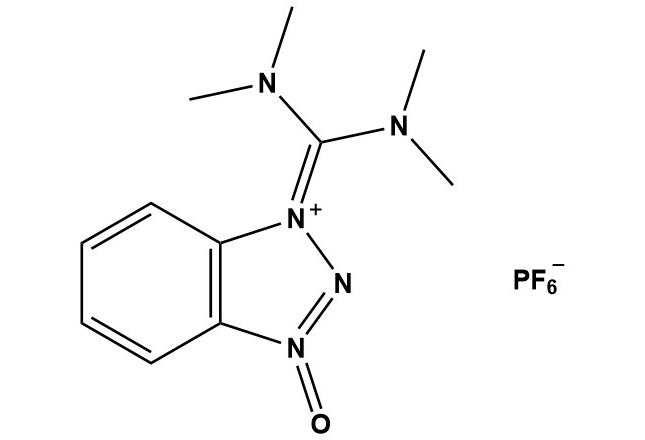
| Synonym: | 1-[Bis(dimethylamino)methylene]-1H-benzotriazolium 3-oxide hexafluorophosphate |
| CAS #: | 94790-37-1 |
| Molecular Formula: | C11H16N5O•F6P |
| Molecular Weight: | 379.2 |
| HBTU (CAS #: 94790-37-1) is one of the most widely utilized and commercially successful peptide coupling reagents in modern organic synthesis. Since its introduction in the early 1990s, HBTU has become the workhorse reagent for amide bond formation in both solid-phase and solution-phase peptide synthesis. Its exceptional balance of reactivity, cost-effectiveness, commercial availability, and broad applicability has made it the standard coupling reagent in academic laboratories and industrial peptide manufacturing worldwide. 1. Introduction The development of HBTU represents a landmark achievement in peptide chemistry, addressing the critical need for efficient, reliable, and cost-effective coupling reagents. HBTU was developed in the late 1980s and early 1990s as part of a family of benzotriazole-based uronium and phosphonium salts designed to overcome the limitations of earlier coupling methods such as dicyclohexylcarbodiimide (DCC) and its water-soluble analogue EDC. HBTU belongs to the class of uronium-type coupling reagents and incorporates 1-hydroxybenzotriazole (HOBt) as an integral component of its structure. The combination of rapid activation kinetics, suppressed racemization, and compatibility with standard protecting group strategies has established HBTU as the most popular peptide coupling reagent globally. Its widespread adoption has been further facilitated by competitive pricing, excellent shelf stability, and availability from numerous commercial suppliers. 2. Chemical Structure and Properties 2.1 Molecular Structure HBTU consists of a benzotriazole moiety connected to a tetramethyluronium group, with a hexafluorophosphate counterion. The molecular formula is C11H16N5O•F6P, with a molecular weight of 379.24 g/mol. Key structural features include: • The benzotriazole core, which serves both as the leaving group and as an additive to suppress racemization through formation of highly reactive HOBt active esters • The tetramethyluronium substituent, which provides electrophilic activation of the carboxylic acid • The hexafluorophosphate anion, which ensures excellent solubility in organic solvents and provides a weakly coordinating, non-nucleophilic counterion 2.2 Physical Properties Appearance: White to off-white crystalline powder Molecular Weight: 379.2 g/mol Melting Point: Decomposes above 150°C Solubility: Soluble in DMF, NMP, DCM, THF; sparingly soluble in acetonitrile Storage Conditions: Store at room temperature in tightly sealed container, desiccated Stability: Stable for years when stored properly; hygroscopic 3. Mechanism of Action 3.1 General Coupling Mechanism The mechanism of HBTU-mediated peptide coupling proceeds through a well-established pathway involving multiple reactive intermediates: Step 1: Deprotonation and Initial Activation In the presence of a tertiary amine base (typically DIPEA, N-methylmorpholine, or triethylamine), the carboxylic acid is deprotonated to form the carboxylate anion. This nucleophilic species attacks the electrophilic carbon of HBTU’s uronium group, leading to displacement and formation of a reactive O-acylisourea intermediate. Step 2: Formation of the HOBt Active Ester The O-acylisourea intermediate undergoes rapid rearrangement or displacement by hydroxybenzotriazole (HOBt), which is liberated during the initial activation step. This produces the key reactive intermediate: the HOBt active ester. The HOBt ester exhibits enhanced reactivity compared to the parent carboxylic acid while maintaining sufficient stability to minimize side reactions. Step 3: Aminolysis and Product Formation The amine nucleophile attacks the carbonyl carbon of the HOBt ester, displacing HOBt as a leaving group and forming the desired amide bond. The excellent leaving group ability of HOBt, combined with the electron-withdrawing effect of the triazole ring, ensures efficient coupling even with moderately hindered substrates. 3.2 The Uronium vs. Guanidinium Debate An important mechanistic consideration is the so-called uronium/guanidinium controversy. Early studies proposed that HBTU and related reagents exist as uronium salts. However, subsequent investigations revealed that in solution, particularly in the presence of base, these compounds may exist in equilibrium with their guanidinium tautomers. The practical implications of this tautomerism are minimal, as both forms are capable of activating carboxylic acids efficiently. 3.3 Racemization Suppression HBTU provides good, though not exceptional, suppression of racemization during peptide coupling. Racemization occurs primarily through base-catalyzed abstraction of the α-proton, leading to enolization and loss of stereochemical integrity. HBTU minimizes racemization through several mechanisms: • Rapid formation and consumption of the HOBt active ester reduces the lifetime of potentially racemizable intermediates • The benzotriazole moiety provides moderate electron-withdrawing effects that reduce the acidity of the α-proton • Use of weaker bases (DIPEA) rather than stronger bases reduces base-catalyzed epimerization However, it is important to note that HBTU exhibits slightly higher racemization rates compared to more advanced reagents like HATU, particularly for C-terminal cysteine, histidine, and other racemization-prone amino acids. 4. Applications in Synthesis 4.1 Solid-Phase Peptide Synthesis HBTU is the most widely used coupling reagent for Fmoc-based solid-phase peptide synthesis (SPPS). Its applications in SPPS include: Standard Peptide Synthesis: HBTU provides excellent yields for routine peptide couplings and is the default choice in most automated peptide synthesizers. Typical coupling times range from 30-60 minutes at room temperature. High-Throughput Synthesis: The cost-effectiveness and reliability of HBTU make it ideal for parallel peptide synthesis and library generation where large quantities of coupling reagent are required. Long Peptide Sequences: HBTU has been successfully employed in the synthesis of peptides exceeding 50 amino acids, though difficult sequences may require supplementation with more reactive reagents or double coupling protocols. Resin Compatibility: HBTU is compatible with all standard SPPS resins including Wang, Rink amide, 2-chlorotrityl, and other commonly used supports. 4.2 Solution-Phase Peptide Synthesis While HBTU is predominantly used in solid-phase synthesis, it also finds application in solution-phase peptide chemistry: Fragment Coupling: HBTU can be employed for the coupling of protected peptide fragments in solution, though yields may be lower compared to solid-phase methods due to solubility issues and increased side reactions. Small-Scale Syntheses: For short peptides and peptide derivatives, HBTU-mediated solution-phase synthesis offers a convenient alternative to SPPS. Convergent Synthesis: HBTU can facilitate the assembly of complex peptides through convergent strategies involving the coupling of protected segments. 4.3 Non-Peptide Applications Beyond peptide chemistry, HBTU has found utility in diverse synthetic applications: Amide Bond Formation: HBTU is widely used for general amide synthesis in medicinal chemistry, including the preparation of drug candidates and pharmaceutical intermediates. Natural Product Synthesis: HBTU enables efficient amide bond formation in the total synthesis of complex natural products containing amide linkages. Bioconjugation: HBTU facilitates the conjugation of carboxylic acid-containing molecules to amine-bearing biomolecules, including proteins, antibodies, and oligonucleotides. Macrocyclization: HBTU can promote intramolecular cyclization reactions, though it is generally less effective than HATU for challenging macrolactamizations. Ester Synthesis: Under appropriate conditions, HBTU can activate carboxylic acids for esterification reactions with alcohols. 5. Practical Considerations 5.1 Standard Reaction Conditions Typical HBTU coupling reactions employ the following conditions: • Solvent: DMF (most common), NMP, or DCM for less polar substrates • Base: DIPEA (2-4 equivalents), N-methylmorpholine, or triethylamine • HBTU loading: 1.0-1.5 equivalents relative to the carboxylic acid • Optional HOBt additive: 0-1.0 equivalents (often omitted as HBTU generates HOBt in situ) • Temperature: Room temperature (20-25°C) • Reaction time: 30 minutes to 2 hours • Atmosphere: Inert (nitrogen or argon) to prevent oxidation and moisture contamination 5.2 Optimization Strategies For challenging couplings or to maximize yields, several optimization approaches can be employed: Pre-activation: Pre-activating the carboxylic acid with HBTU and base for 2-5 minutes before adding the amine component can improve coupling efficiency. Extended Reaction Times: Difficult couplings may benefit from longer reaction times (2-4 hours) or elevated temperatures (up to 40°C), though this increases racemization risk. Double Coupling: In SPPS, performing two sequential coupling reactions with fresh reagents can drive incomplete couplings to completion. Excess Reagents: Using 2-3 equivalents of HBTU and activated amino acid (relative to resin loading) can improve yields in difficult cases. Microwave Assistance: Microwave irradiation can significantly accelerate HBTU-mediated couplings and is increasingly used in automated SPPS instruments. 6. Advantages and Disadvantages 6.1 Advantages HBTU offers numerous advantages that have established it as the most popular peptide coupling reagent: • Cost-Effectiveness: HBTU is significantly less expensive than advanced coupling reagents like HATU, making it economical for large-scale synthesis and high-throughput applications. • Excellent Shelf Stability: When stored properly, HBTU remains active for years, reducing waste and simplifying inventory management. • Broad Applicability: HBTU provides good to excellent yields for most standard peptide couplings and is compatible with all common protecting group strategies. • Fast Reaction Rates: Most couplings reach completion within 30-60 minutes at room temperature. • Well-Established Protocols: Decades of use have resulted in thoroughly optimized protocols for virtually any peptide synthesis application. • Easy Workup: In solution-phase synthesis, HBTU byproducts are generally easy to remove through aqueous washes or chromatography. • Compatibility with Automation: HBTU is the standard coupling reagent in commercial peptide synthesizers due to its reliability and ease of handling. 6.2 Disadvantages and Limitations Despite its many strengths, HBTU has several notable limitations: • Racemization: HBTU exhibits higher racemization rates than advanced reagents like HATU and PyBOP, particularly for C-terminal cysteine, histidine, and other sensitive amino acids. • Lower Efficiency for Hindered Couplings: HBTU may give incomplete coupling with sterically demanding amino acids such as N-methylated amino acids or highly β-branched residues. • Guanidinium Formation: Reaction of HBTU with primary amines can lead to tetramethylguanidinium byproducts, particularly if the amine is added directly to HBTU solutions. • Fluoride Release: The hexafluorophosphate counterion can slowly hydrolyze to release fluoride, which may cause issues with silica-based resins or silicon-containing protecting groups. • HOBt Explosion Hazard: Pure HOBt, which is liberated during HBTU-mediated couplings, is potentially explosive when dry. While the quantities generated in typical reactions are not hazardous, this should be considered when scaling up. • Limited Reactivity for Very Difficult Couplings: For extremely challenging transformations such as large macrocyclizations or very hindered substrates, more reactive reagents may be necessary. 7. Comparison with Other Coupling Reagents HBTU Advantages: Low cost, widely available, reliable, fast reactions, good stability Disadvantages: Higher racemization, less effective for very hindered couplings Best Use Case: Standard peptide synthesis, automated SPPS, cost-sensitive applications HATU Advantages: Minimal racemization, excellent for hindered substrates, faster reactions Disadvantages: More expensive, less widely available Best Use Case: Difficult couplings, racemization-prone amino acids, macrocyclization PyBOP Advantages: Low racemization, good for hindered couplings, different counterion Disadvantages: More expensive than HBTU, slower than HATU Best Use Case: Alternative to HBTU when lower racemization needed DIC/DCC Advantages: Very low cost, simple chemistry Disadvantages: Significant racemization, requires HOBt/HOAt additive, difficult workup Best Use Case: Budget-constrained applications, non-peptide amide synthesis EDC Advantages: Water-soluble, low cost, water-soluble byproducts Disadvantages: Requires HOBt/HOAt, slower reactions, less efficient than uronium salts Best Use Case: Aqueous or mixed solvent systems, bioconjugation COMU Advantages: Very low racemization, excellent for hindered couplings, no HOBt explosion risk Disadvantages: Expensive, less widely used Best Use Case: Alternative to HATU, sensitive amino acids 8. Side Reactions and Troubleshooting 8.1 Common Side Reactions Racemization: HBTU-mediated couplings can exhibit measurable racemization, particularly with C-terminal cysteine, histidine, asparagine, and glutamine. Minimized by using lower temperatures, weaker bases, and shorter reaction times. Guanidinium Formation: Direct reaction of HBTU with primary amines leads to N,N,N’,N’-tetramethylguanidinium byproducts. Avoided by pre-activating the acid with HBTU and base before adding the amine. Aspartimide Formation: Peptides containing aspartate residues can undergo intramolecular cyclization to form aspartimides. Minimized by using appropriate side-chain protecting groups (tert-butyl preferred over benzyl) and avoiding strongly basic conditions. N-Terminal Modifications: Free N-terminal amines may undergo unwanted acylation or formylation as side reactions. Proper protection strategies prevent this issue. Incomplete Coupling: Hindered amino acids or sterically demanding substrates may not react to completion. Addressed through double coupling, extended reaction times, or switching to more reactive coupling reagents. 8.2 Troubleshooting Guide Low coupling yield Possible Cause: Degraded HBTU, insufficient activation, hindered substrates Solution: Use fresh HBTU, extend reaction time, perform double coupling, switch to HATU Racemization Possible Cause: Sensitive amino acid, strong base, prolonged reaction time Solution: Lower temperature, use DIPEA, reduce reaction time, switch to HATU or PyBOP Guanidinium impurities Possible Cause: Direct HBTU-amine reaction Solution: Pre-activate acid with HBTU before adding amine Aspartimide formation Possible Cause: Aspartate side-chain reactivity Solution: Use tert-butyl protecting group, minimize base concentration Incomplete coupling in SPPS Possible Cause: Aggregation, steric hindrance, low resin swelling Solution: Add chaotropic agents, perform double coupling, increase temperature Poor solubility Possible Cause: Polar substrates in DCM, non-polar substrates in DMF Solution: Adjust solvent system, use co-solvents, increase concentration 9. Recent Developments and Future Directions While HBTU is a mature technology, several recent developments have expanded its utility: Microwave-Assisted Synthesis: The combination of HBTU with microwave irradiation has enabled ultra-fast peptide synthesis, with coupling times reduced to 1-5 minutes while maintaining high yields. Flow Chemistry: HBTU has been successfully employed in continuous-flow peptide synthesis systems, offering advantages for manufacturing and process intensification. Automation: Modern peptide synthesizers increasingly incorporate HBTU as the standard coupling reagent, with optimized protocols for both research and production scales. Green Chemistry Initiatives: Research into recyclable coupling reagents and less toxic alternatives continues, though HBTU’s performance-to-cost ratio ensures it will remain widely used for the foreseeable future. Hybrid Approaches: Combining HBTU for routine couplings with more reactive or selective reagents (like HATU) for difficult steps has become common practice in complex peptide synthesis. 10. Conclusion HBTU has earned its position as the most widely used peptide coupling reagent through a unique combination of effectiveness, reliability, cost-efficiency, and commercial availability. While newer reagents like HATU offer superior performance in specific applications, HBTU remains the optimal choice for the vast majority of peptide synthesis applications, from academic research to industrial manufacturing. The reagent’s excellent balance of coupling efficiency, suppressed racemization, rapid reaction rates, and affordability ensures it will continue to dominate peptide chemistry for years to come. Understanding the proper use of HBTU, including its mechanism, optimization strategies, and limitations, enables chemists to fully leverage this powerful tool for amide bond formation. For practitioners of peptide chemistry, HBTU represents the gold standard against which other coupling reagents are measured. Its proven track record in thousands of publications and countless industrial applications demonstrates that sometimes the most successful reagent is not necessarily the most advanced, but rather the one that best combines performance, practicality, and economy. References 1. Dourtoglou, V., et al. (1984). O-Benzotriazolyl-N,N,N’,N’-tetramethyluronium hexafluorophosphate as coupling reagent for the synthesis of peptides. Synthesis. 2. Knorr, R., et al. (1989). New coupling reagents in peptide chemistry. Tetrahedron Letters. 3. El-Faham, A., and Albericio, F. (2011). Peptide coupling reagents, more than a letter soup. Chemical Reviews. 4. Valeur, E., and Bradley, M. (2009). Amide bond formation: beyond the myth of coupling reagents. Chemical Society Reviews. 5. Montalbetti, C.A., and Falque, V. (2005). Amide bond formation and peptide coupling. Tetrahedron. 6. Han, S.-Y., and Kim, Y.-A. (2004). Recent development of peptide coupling reagents in organic synthesis. Tetrahedron. |
|
HBTU
For Research & Development use only. Not for testing and/or use on humans.