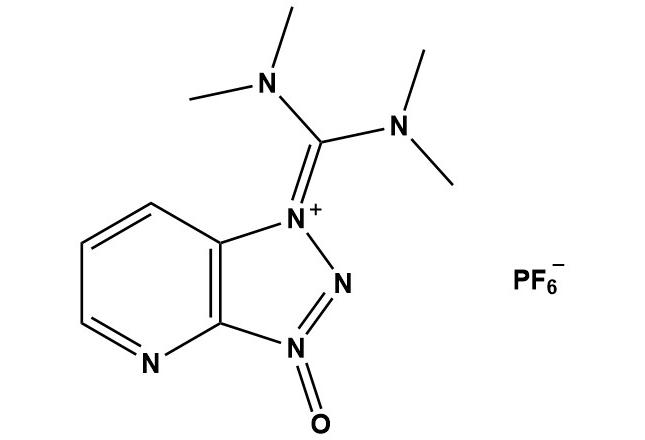
| Synonym: | 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxide hexafluorophosphate |
| CAS #: | 148893-10-1 |
| Molecular Formula: | C10H15N6O•F6P |
| Molecular Weight: | 380.2 |
| HATU (CAS #: 148893-10-1) is one of the most important and widely used peptide coupling reagents in modern synthetic organic chemistry. Since its introduction in the 1990s, HATU has become a staple reagent for amide bond formation, particularly in peptide synthesis, due to its exceptional efficiency, minimal racemization, and compatibility with a wide range of functional groups. 1. Introduction The development of efficient peptide coupling reagents has been a critical advancement in organic synthesis, enabling the construction of complex peptides and proteins for pharmaceutical, biological, and materials science applications. HATU was developed by Carpino and colleagues as part of a series of uronium and phosphonium-based coupling reagents designed to overcome the limitations of earlier coupling methods.HATU belongs to the family of 1-hydroxy-7-azabenzotriazole (HOAt)-derived coupling reagents and represents a significant improvement over earlier reagents such as dicyclohexylcarbodiimide (DCC) and benzotriazol-1-yl-oxytripyrrolidinophosphonium hexafluorophosphate (PyBOP). The introduction of HATU addressed key challenges in peptide synthesis, including racemization suppression, coupling efficiency with hindered amino acids, and the minimization of side reactions. 2. Chemical Structure and Properties 2.1 Molecular Structure HATU consists of a HOAt-derived triazolopyridinium core substituted with a bis(dimethylamino)methylene group, paired with a hexafluorophosphate counterion. The molecular formula is C10H15N6O•F6P, with a molecular weight of 380.2 g/mol. Key structural features include: • The 1,2,3-triazolo[4,5-b]pyridinium N-oxide core, which provides enhanced reactivity compared to benzotriazole-based reagents • The bis(dimethylamino)methylene substituent, which serves as the leaving group during coupling reactions • The hexafluorophosphate anion, which ensures solubility in organic solvents and provides a non-nucleophilic counterion 2.2 Physical Properties Appearance: White to off-white crystalline solid Molecular Weight: 380.2 g/mol Melting Point: Decomposes above 140°C Solubility: Soluble in DMF, NMP, DCM, acetonitrile; insoluble in water Storage Conditions: Store at 2-8°C in tightly sealed container under inert atmosphere 3. Mechanism of Action 3.1 General Coupling Mechanism The mechanism of HATU-mediated peptide coupling proceeds through several key steps: Step 1: Activation of the Carboxylic Acid In the presence of a tertiary amine base (typically DIPEA or N-methylmorpholine), HATU reacts with the carboxylic acid to form a highly reactive O-acylisourea intermediate. This activation step is facilitated by deprotonation of the carboxylic acid and nucleophilic attack on the electrophilic carbon of the bis(dimethylamino)methylene group. Step 2: Formation of the Active Ester The O-acylisourea intermediate rapidly rearranges or undergoes displacement by HOAt (which is released during the initial activation step) to form a highly reactive HOAt active ester. This ester is significantly more reactive than the corresponding HOBt (1-hydroxybenzotriazole) esters formed with older coupling reagents, contributing to HATU’s superior coupling efficiency. Step 3: Aminolysis and Amide Bond Formation The nucleophilic amine component attacks the carbonyl carbon of the active ester, leading to displacement of HOAt and formation of the desired amide bond. The enhanced electrophilicity of the HOAt ester, combined with the excellent leaving group ability of HOAt, ensures rapid and efficient coupling even with sterically hindered substrates. 3.2 Racemization Suppression One of the most significant advantages of HATU is its ability to minimize racemization during peptide coupling. Racemization typically occurs through base-catalyzed deprotonation of the α-carbon adjacent to the carbonyl group, leading to enolization and loss of stereochemical integrity. HATU suppresses racemization through several mechanisms: • The rapid formation and consumption of the HOAt active ester minimizes the lifetime of reactive intermediates that could undergo racemization • The N-oxide functionality in the HOAt moiety provides electron-withdrawing effects that destabilize enolate formation • The use of weaker bases (such as DIPEA) compared to stronger bases required for older coupling methods reduces the extent of base-catalyzed racemization 4. Applications in Synthesis 4.1 Peptide Synthesis HATU is most extensively used in solid-phase peptide synthesis (SPPS) and solution-phase peptide synthesis. Its applications include: Fmoc-Based Solid-Phase Peptide Synthesis: HATU is compatible with the Fmoc protection strategy and is often the coupling reagent of choice for difficult couplings, particularly those involving hindered or β-branched amino acids such as valine, isoleucine, and threonine. Boc-Based Synthesis: While less common due to the predominance of Fmoc chemistry, HATU can also be used in Boc-based peptide synthesis, though care must be taken to avoid acidic conditions that could protonate the N-oxide functionality. Cyclic Peptide Synthesis: HATU is effective for macrolactamization reactions required in the synthesis of cyclic peptides, including head-to-tail, side-chain-to-tail, and head-to-side-chain cyclizations. Peptoid Synthesis: HATU has been successfully employed in the synthesis of peptoids (N-substituted glycine oligomers), where its efficiency is particularly valuable for coupling secondary amines. 4.2 Non-Peptide Applications Beyond peptide chemistry, HATU has found utility in a variety of synthetic transformations: Natural Product Synthesis: HATU is frequently employed in the total synthesis of natural products containing amide bonds, particularly in cases where conventional coupling methods fail or lead to extensive racemization. Medicinal Chemistry: In the synthesis of drug candidates and pharmaceutical intermediates, HATU enables the efficient construction of amide bonds in complex molecular architectures. Macrocyclization: HATU is effective for the synthesis of macrocyclic lactams, including large-ring systems that are challenging to close using other methods. Polymer Synthesis: HATU has been used in the preparation of polyamides and other nitrogen-containing polymers where controlled amide bond formation is required. 5. Practical Considerations 5.1 Typical Reaction Conditions Standard HATU coupling reactions are typically performed under the following conditions: • Solvent: DMF, NMP, or DCM (for less polar substrates) • Base: DIPEA (Hünig’s base) or N-methylmorpholine, 2-4 equivalents • HATU loading: 1.0-1.5 equivalents relative to the carboxylic acid component • Temperature: Room temperature to 0°C (lower temperatures for racemization-prone substrates) • Reaction time: 30 minutes to 4 hours (varies with substrate reactivity) • Atmosphere: Inert (nitrogen or argon) to prevent oxidation and hydrolysis 5.2 Optimization Strategies For challenging couplings, several optimization strategies can improve yields: Pre-activation Protocol: The carboxylic acid can be pre-activated with HATU and base for 2-5 minutes before addition of the amine component, allowing complete formation of the active ester. Multiple Coupling Cycles: In SPPS, difficult couplings can be driven to completion by performing sequential coupling reactions with fresh reagents. Additive Use: HOAt can be added as a co-additive (0.2-0.5 eq.) to enhance coupling efficiency, though this is typically unnecessary with HATU. Temperature Adjustment: Lowering the reaction temperature to 0-5°C can reduce racemization for sensitive amino acids, while slightly elevated temperatures (30-40°C) may improve coupling rates for hindered substrates. 6. Advantages and Disadvantages 6.1 Advantages HATU offers several significant advantages over alternative coupling reagents: • Minimal Racemization: HATU exhibits exceptionally low levels of racemization, making it suitable for coupling C-terminal amino acids prone to epimerization (such as cysteine, histidine, and phenylalanine). • High Coupling Efficiency: The reagent provides excellent yields even with sterically hindered amino acids and secondary amines. • Rapid Reaction Rates: Coupling reactions with HATU typically reach completion within 30-60 minutes at room temperature. • Broad Substrate Scope: HATU is compatible with a wide range of functional groups and protecting group strategies. • Solubility: The reagent exhibits excellent solubility in common peptide synthesis solvents, facilitating homogeneous reaction conditions. • Clean Reaction Profile: HATU typically produces minimal side products, and the HOAt and HATU-derived byproducts are easily removed during workup. 6.2 Disadvantages and Limitations Despite its many advantages, HATU has several limitations: • Cost: HATU is significantly more expensive than traditional coupling reagents such as DCC or EDC, which may be prohibitive for large-scale applications. • Moisture Sensitivity: The hygroscopic nature of HATU requires careful storage and handling to maintain reagent activity. • Potential for Guanidine Formation: Under certain conditions, particularly with excess base or prolonged reaction times, HATU can react with primary amines to form guanidinium species as a side product. • Fluoride Contamination: The hexafluorophosphate counterion can hydrolyze to release fluoride ions, which may interfere with silicon-based protecting groups or peptide cleavage from silica-based resins. 7. Comparison with Other Coupling Reagents HATU Advantages: Minimal racemization, fast, efficient with hindered substrates Disadvantages: Expensive, moisture sensitive, potential guanidine formation Best Use Case: Difficult peptide couplings, C-terminal sensitive residues HBTU Advantages: Less expensive, widely available, good general performance Disadvantages: More racemization than HATU, slower reactions Best Use Case: Standard peptide synthesis, cost-sensitive applications DCC/DIC Advantages: Inexpensive, effective for simple couplings Disadvantages: Significant racemization, difficult purification of urea byproducts Best Use Case: Simple amide formations, non-peptide applications EDC Advantages: Water-soluble, easy workup, low cost Disadvantages: Requires co-additives (HOBt/HOAt), slower than uronium reagents Best Use Case: Aqueous couplings, bioconjugation PyBOP Advantages: Low racemization, good for hindered couplings Disadvantages: Slower than HATU, less effective for very difficult couplings Best Use Case: General peptide synthesis, alternative to HBTU 8. Side Reactions and Troubleshooting 8.1 Common Side Reactions Guanidinium Formation: Reaction of HATU with primary amines can lead to N,N,N’,N’-tetramethylguanidinium species. This side reaction is minimized by adding HATU to a mixture of the carboxylic acid and base, rather than adding the amine to pre-formed HATU solutions. Aspartimide Formation: In peptides containing aspartic acid residues, HATU-mediated couplings can promote aspartimide formation through intramolecular cyclization. This can be minimized by using protecting groups that reduce nucleophilicity of the aspartate side chain (e.g., tert-butyl ester rather than benzyl ester). N-Terminal Acetylation: Free N-terminal amines can undergo acetylation as a side reaction. Using appropriate N-terminal protection strategies prevents this issue. 8.2 Troubleshooting Guide Low coupling yield Possible Cause: Moisture-degraded HATU, insufficient activation time Solution: Use fresh HATU, extend pre-activation time, perform double coupling Racemization detected Possible Cause: High temperature, strong base, prolonged reaction time Solution: Lower temperature to 0°C, use DIPEA instead of stronger bases, reduce reaction time Guanidinium byproducts Possible Cause: Direct reaction of HATU with amine Solution: Pre-activate acid with HATU before adding amine, ensure proper order of addition Difficult purification Possible Cause: HOAt or HATU-derived byproducts co-eluting Solution: Perform aqueous washes with dilute acid/base, adjust chromatography conditions Aspartimide formation Possible Cause: Aspartate side-chain reactivity Solution: Use tert-butyl protection for Asp side chain, minimize base concentration, lower temperature 9. Recent Developments and Future Directions Recent research has focused on developing more sustainable and cost-effective alternatives to HATU while maintaining its excellent performance characteristics. Several trends are emerging: Green Chemistry Initiatives: Efforts to develop environmentally benign coupling reagents have led to the exploration of uronium salts with less problematic counterions and recyclable coupling reagents. Immobilized Coupling Reagents: Polymer-supported versions of HATU and related reagents have been developed to facilitate purification and enable continuous-flow peptide synthesis. Microwave-Assisted Synthesis: The use of microwave irradiation in combination with HATU has enabled even faster coupling reactions and improved yields for difficult sequences. Automated Peptide Synthesis: HATU remains a reagent of choice for high-throughput automated peptide synthesizers, where its reliability and efficiency are crucial for producing peptide libraries. 10. Conclusion HATU has established itself as one of the premier coupling reagents in modern synthetic chemistry, particularly for peptide synthesis. Its combination of high coupling efficiency, minimal racemization, broad functional group tolerance, and rapid reaction kinetics makes it indispensable for both research and industrial applications. While cost and moisture sensitivity present challenges, the superior performance of HATU often justifies these drawbacks, especially for complex or high-value synthetic targets. As peptide therapeutics continue to grow in importance and synthetic methodologies advance, HATU is likely to remain a critical tool for chemists engaged in amide bond formation. Understanding its mechanism, optimizing reaction conditions, and recognizing potential pitfalls enables practitioners to fully leverage the capabilities of this powerful reagent. References 1. Carpino, L.A., et al. (1994). The uronium/guanidinium peptide coupling reagents: Finally the true uronium salts. Tetrahedron Letters. 2. El-Faham, A., and Albericio, F. (2011). Peptide coupling reagents, more than a letter soup. Chemical Reviews. 3. Valeur, E., and Bradley, M. (2009). Amide bond formation: beyond the myth of coupling reagents. Chemical Society Reviews. 4. Montalbetti, C.A., and Falque, V. (2005). Amide bond formation and peptide coupling. Tetrahedron. |
|