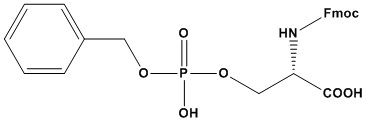
| Synonym: | N-α-Fmoc-O-benzyl-L-phosphoserine |
| CAS #: | 158171-14-3 |
| Molecular Formula: | C25H24NO8P |
| Molecular Weight: | 497.4 |
| Fmoc-Ser(HPO3Bzl)-OH is an excellent building block for the preparation of phosphopeptides. This derivative can be introduced using standard activation methods, such as PyBOP and TBTU. The monoprotected phosphoserine residue once incorporated is stable to piperidine. Using this reagent, even peptides containing multiple phosphorylation sites can be prepared efficiently by standard Fmoc SPPS methods. β-piperidinylalanine formation has been shown to occur during Fmoc deprotection of N-terminal Ser(PO(OBzl)OH), particularly under microwave conditions. This side reaction can be eliminated by using cyclohexylamine or DBU just for this Fmoc deprotection step . References 1. T. Wakamiya, et al. (1994) Chem. Lett., 1099. 2. P. White & J. Beythien in “Innovations & Perspectives in Solid Phase Synthesis and Combinatorial Libraries, 4th International Symposium”, Mayflower Scientific Ltd., Birmingham, 1996, pp. 557. 3. T. J. Attard, et al. (2009) Int. J. Pept. Res. Ther., 15, 69. 4. J.W. Perich et al. Lett. Pept. Sci. 6, 91, (1999). 5. H. Schmid et al. Innovation Perspect. Solid Phase Synt. Comb. Libr., 4th Int. Symp., 525, (1995). |
|
Fmoc-Ser(HPO3Bzl)-OH
For Research & Development use only. Not for testing and/or use on humans.