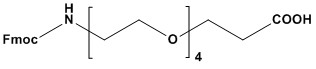
| Synonym: | Fmoc-15-amino-4,7,10,13-tetraoxapentadecacanoic acid |
| CAS #: | 557756-85-1 |
| Molecular Formula: | C26H33NO8 |
| Molecular Weight: | 487.5 |
| Fmoc-NH-PEG4-CH2CH2COOH, also known as Fmoc-NH-PEG₄-COOH or Fmoc-N-amido-PEG4-acid is a bifunctional polyethylene glycol (PEG) derivative widely employed in bioconjugation, peptide synthesis, and drug delivery systems. With the CAS number 557756-85-1, this compound features an Fmoc (9-fluorenylmethyloxycarbonyl)-protected amine at one end and a terminal carboxylic acid at the other, connected by a hydrophilic PEG4 spacer (four ethylene glycol units). The PEG spacer imparts solubility-enhancing properties, making it valuable for applications requiring biocompatibility and reduced immunogenicity. 1. Chemical Structure and Functional Components The power of Fmoc-NH-PEG4-CH2CH2COOH lies in its modular structure, which consists of three distinct functional parts: 1.1 Fmoc (9-fluorenylmethyloxycarbonyl) Group This is a protecting group for the amine. The Fmoc group is famously base-labile, meaning it is stable under acidic and neutral conditions but can be cleanly and rapidly removed by treatment with a mild base, such as 20% piperidine in DMF. This property is the cornerstone of its utility in solid-phase peptide synthesis (SPPS), where it allows for the sequential addition of amino acids without affecting acid-labile protecting groups on the peptide side chains. 1.2 PEG₄ (Tetraethylene Glycol) Spacer This is the central linker or spacer unit. It is a discrete, monodisperse polyethylene glycol chain consisting of four repeating oxyethylene units. The PEG spacer imparts several highly desirable properties: • Hydrophilicity: It significantly increases the aqueous solubility of the molecule it’s attached to, which is crucial for preventing the aggregation of peptides, proteins, or drug conjugates in biological media. • Flexibility and Spacing: It provides a flexible, defined-length spacer arm (approximately 17.7 Å) that separates the two conjugated molecules, minimizing steric hindrance and allowing each component to maintain its native conformation and function. • Biocompatibility: PEG is well-known for being non-toxic and non-immunogenic, making it ideal for in vivo applications. It can help “shield” the conjugate from the immune system and proteolytic enzymes. 1.3 COOH (Carboxylic Acid) Group This is the second reactive handle of the linker. The terminal carboxylic acid can be readily activated using standard carbodiimide chemistry (e.g., with EDC, DCC) or other coupling reagents (e.g., HBTU, HATU) to form a stable amide bond with a primary or secondary amine on a target molecule, such as a peptide, protein, antibody, or functionalized surface. 2. Key Applications The unique combination of these three components makes Fmoc-NH-PEG4-CH2CH2COOH a versatile tool in several areas of biochemical research and development. 2.1 Solid-Phase Peptide Synthesis (SPPS) In SPPS, this linker is used to introduce a hydrophilic spacer within a peptide sequence or to functionalize the N-terminus. By coupling the carboxylic acid end to the free amine of a growing peptide chain, a PEGylated N-terminus is created. After the synthesis is complete, the Fmoc group can be removed to expose a primary amine, which can then be used for subsequent labeling with a fluorophore, biotin, or another molecule. 2.2 Bioconjugation and PEGylation This molecule is a classic example of a linker used for PEGylation—the process of attaching PEG chains to molecules. Its heterobifunctional nature allows for a controlled, two-step conjugation strategy: • Step 1: The carboxylic acid is activated and reacted with an amine on Molecule A (e.g., an antibody or protein). • Step 2: The Fmoc group is removed using a base, revealing a new amine. • Step 3: This newly exposed amine is then used to react with an activated functional group (like an NHS ester) on Molecule B (e.g., a small molecule drug or a fluorescent dye). This linker is particularly valuable for creating antibody-drug conjugates (ADCs), where controlling the drug-to-antibody ratio (DAR) and ensuring the solubility and stability of the final product are critical. 2.3 Surface Modification and Material Science Fmoc-NH-PEG4-CH2CH2COOH is used to functionalize surfaces like gold nanoparticles, quantum dots, and microarrays. The carboxylic acid can be coupled to an amine-functionalized surface. The PEG chain then forms a hydrophilic layer that resists non-specific protein adsorption, a critical feature for developing reliable biosensors and diagnostic devices. After Fmoc deprotection, the terminal amine provides a site for the covalent immobilization of capture probes like antibodies or DNA oligonucleotides. 3. Advantages and Disadvantages 3.1 Advantages • Orthogonal Reactivity: The base-labile Fmoc group and the activatable carboxylic acid provide excellent chemical orthogonality, allowing for precise, stepwise reactions without unintended side reactions. This is a major advantage over linkers with two identical reactive groups. • Monodispersity: Unlike traditional polydisperse PEG polymers, this linker has a precisely defined length and molecular weight. This ensures homogeneity in the final conjugate, leading to more predictable behavior, simplified purification, and easier characterization—a requirement for therapeutic agents. • Enhanced Biophysical Properties: The PEG spacer improves the solubility, in vivo half-life, and stability of bioconjugates while reducing their immunogenicity. • Versatility: It can be used to link a vast array of molecules, from small organic compounds to large proteins and material surfaces. 3.2 Disadvantages • Cost: As a highly pure, monodisperse, and multi-functionalized reagent, it is significantly more expensive than simple hydrocarbon linkers or bulk, polydisperse PEG polymers. • Susceptibility to Oxidation: The ether linkages in the PEG backbone can be susceptible to auto-oxidation over long periods or under harsh conditions, though this is rarely an issue under standard laboratory use and storage. 4. Handling and Storage For optimal stability, Fmoc-NH-PEG4-CH2CH2COOH should be stored under cold and dry conditions, typically at -20°C, and protected from light. It is recommended to keep it under an inert atmosphere (e.g., Argon or Nitrogen) to minimize exposure to moisture and oxygen, which could degrade the compound over time. Before use, the container should be allowed to warm to room temperature before opening to prevent condensation of moisture onto the product. It is soluble in common organic solvents like DMF, DMSO, and DCM. Fmoc-NH-PEG4-CH2CH2COOH (CAS 557756-85-1) is a sophisticated and indispensable molecular linker in modern biotechnology. Its well-defined structure, combining an orthogonal Fmoc-protected amine, a biocompatible PEG spacer, and a reactive carboxylic acid, provides chemists and biologists with a powerful tool for constructing complex, functional biomolecules. Its contributions are particularly notable in the development of advanced therapeutics like peptide drugs and antibody-drug conjugates, as well as in the creation of high-performance diagnostic and material science platforms. References: 1. Overview of PEG Linkers 2. Overview of Polyethylene Glycol (PEG) 3. Polyethylene Glycol 4. PEGylation 5. PEGylation – an overview 6. PEGylation, successful approach to drug delivery 7. Peptide Synthesis 8. Overview of Solid Phase Synthesis 9. Fmoc Solid Phase Peptide Synthesis 10. Boc Solid Phase Peptide Synthesis |
|
Fmoc-NH-PEG4-CH2CH2COOH
For Research & Development use only. Not for testing and/or use on humans.