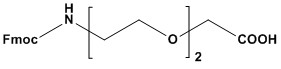
| Synonyms: | Fmoc-8-amino-3,6-dioxaoctanoic acid
Fmoc-AEEA |
| CAS #: | 166108-71-0 |
| Molecular Formula: | C21H23NO6 |
| Molecular Weight: | 385.4 |
| Fmoc-AEEA, also known as Fmoc-8-amino-3,6-dioxaoctanoic acid, is a polyethylene glycol (PEG)-based spacer molecule employed in Fmoc-based solid-phase peptide synthesis (SPPS) and bioconjugation chemistry. The “Fmoc” refers to the 9-fluorenylmethoxycarbonyl group, a base-labile protecting group attached to the amino terminus, while “AEEA” (aminoethoxyethoxyacetic acid) denotes a short PEG-like unit with two ethylene glycol repeats, terminating in a carboxyl group. This structure imparts hydrophilicity and flexibility, making Fmoc- AEEA a valuable tool for enhancing peptide solubility, pharmacokinetics, and functionality. Introduced as a versatile linker, Fmoc-AEEA bridges peptide chains, drugs or biomolecules, improving their biophysical properties. Its compatibility with standard SPPS protocols and its role in therapeutic peptide design, antibody-drug conjugates (ADCs) and diagnostic probes have solidified its importance in modern chemical biology. 1. Chemical Properties • Structure: Fmoc-AEEA features an Fmoc-protected amino group, a diethylene glycol spacer (–NH–CH2–CH2–O–CH2–CH2–O–CH2–), and a terminal acetic acid group (–COOH). The PEG-like backbone is non-reactive and hydrophilic. • Solubility: Highly soluble in polar organic solvents (e.g., DMF, DMSO, NMP) and moderately soluble in water post-Fmoc removal, enhancing peptide solubility in aqueous environments. • Stability: Stable under acidic conditions (e.g., TFA cleavage) but labile to bases (e.g., piperidine), which removes the Fmoc group. The ether linkages resist degradation under typical synthesis conditions. • Purity: Commercial Fmoc-AEEA typically exceeds 97% purity (HPLC), with minimal impurities like free AEEA or Fmoc byproducts. The absence of reactive side chains simplifies its use, while the PEG-like structure mimics the benefits of longer PEGylation without excessive molecular weight. 2. Synthesis of Fmoc-AEEA Fmoc-AEEA is synthesized through a multi-step process starting from diethylene glycol derivatives: 2.1 AEEA Synthesis: • Diethylene glycol (HO–CH2–CH2–O–CH2–CH2–OH) is mono-carboxymethylated with chloroacetic acid under basic conditions (e.g., NaOH), forming HO–CH2–CH2–O–CH2–CH2–O–CH2–COOH. • The terminal hydroxyl is converted to an amine via a Gabriel synthesis or azide reduction, yielding H2N–CH2–CH2–O–CH2–CH2–O–CH2–COOH (AEEA). 2.2 Fmoc Protection: • The amino group of AEEA is reacted with Fmoc-Cl or Fmoc-OSu in a basic aqueous- organic medium (e.g., NaHCO3 in water/dioxane) at 0–5°C, forming Fmoc-AEEA. • pH is maintained at 8–9 to ensure selective protection without side reactions. 2.3 Purification: • The product is precipitated by acidification, extracted (e.g., into ethyl acetate), and purified by recrystallization or chromatography. • NMR, HPLC, and MS confirm purity and structure, ensuring low levels of impurities like Fmoc dimers. 2.4 Challenges: • Incomplete amination or over-alkylation during AEEA synthesis requires optimization. • Residual solvents or salts must be minimized for high-quality applications. 3. Methodology in Peptide Synthesis Fmoc-AEEA is integrated into Fmoc-based SPPS as a linker or spacer: 3.1 Coupling: • The carboxyl group is activated (e.g., with HBTU, DIC/HOBt, or HATU) and coupled to a resin-bound amine or peptide N-terminus. Coupling is efficient due to minimal steric hindrance. 3.2 Fmoc Deprotection: • The Fmoc group is removed with 20–30% piperidine in DMF (5–15 minutes), exposing the amino group for further elongation or conjugation. UV monitoring at 301 nm confirms completion. 3.3 Iterative Use: • Multiple Fmoc-AEEA units can be added to extend spacer length, tailoring the distance between peptide segments or conjugates. 3.4 Cleavage: • The peptide or conjugate is cleaved from the resin with a TFA-based cocktail (e.g., 95% TFA, 2.5% TIS, 2.5% water), leaving the AEEA linker intact in the final product. 4. Applications of Fmoc-AEEA Fmoc-AEEA’s hydrophilic and flexible properties enable a wide range of applications: 4.1 Solid-Phase Peptide Synthesis (SPPS): Fmoc-AEEA is commonly used to introduce spacing elements between peptide segments or between a peptide and a functional group. Benefits include: • Increased solubility of final peptide products • Reduced steric hindrance between functional domains • Enhanced flexibility of the peptide backbone • Improved bioavailability in biological applications 4.2 Bioconjugation: • Creation of peptide-protein conjugates • Development of peptide-small molecule hybrids • Production of peptide-based imaging agents • Synthesis of peptide-drug conjugates • Acts as a linker in ADCs, tethering peptides to drugs, fluorophores or antibodies, reducing steric interference and enhancing biodistribution. 4.3 PEGylation Mimic: • Provides short PEG-like effects (e.g., extended half-life, reduced immunogenicity) without the bulk of longer PEG chains, used in peptide drugs like GLP-1 analogs. 4.4 Cyclic Peptides: • Facilitates cyclization by providing flexibility between reactive termini, improving yields in drug candidates like somatostatin analogs. 4.5 Therapeutic Peptide Design: • Enhances pharmacokinetics and receptor binding in peptides for diabetes, cancer, or inflammation (e.g., insulin derivatives). 4.6 Diagnostics: • Links peptides to imaging agents (e.g., chelators for radiolabeling) in probes for PET or fluorescence imaging. 5. Advantages of Fmoc-AEEA • Hydrophilicity: Enhances solubility and biocompatibility of peptides and conjugates. • Flexibility: Reduces steric hindrance and improves molecular interactions as a non-rigid spacer. • SPPS Compatibility: Integrates seamlessly into Fmoc protocols with standard reagents. • No Side-Chain Protection: Simplifies synthesis compared to amino acids with reactive groups. • Tunable Length: Multiple units can be added to adjust spacer properties. 6. Limitations of Fmoc-AEEA • Cost: More expensive than simple amino acid derivatives due to its PEG-like structure and synthesis complexity. • Limited Rigidity: Unsuitable for applications requiring a rigid linker (e.g., precise molecular positioning). • Molecular Weight: Adds ~160 Da per unit (post-Fmoc removal), potentially undesirable for small peptides. • Stability Concerns: Ether linkages are stable under most conditions but may degrade under extreme oxidative or hydrolytic stress (rare in practice). • Niche Role: Primarily a linker, not a functional residue, limiting its use compared to bioactive amino acids. 7. Recent Advancements • Automated Synthesis: Microwave-assisted SPPS and automated synthesizers (e.g., CEM Liberty) optimize Fmoc-AEEA coupling, improving efficiency. • Hybrid Linkers: Combined with rigid spacers (e.g., aromatic rings) or longer PEG units for tailored conjugate properties. • Green Chemistry: Replacement of DMF with greener solvents (e.g., 2-MeTHF) and reduced TFA use enhance sustainability. • Bioconjugation Innovations: Used in click chemistry-based ADCs, linking peptides to payloads via selective chemistries post-deprotection. • Custom Variants: Derivatives with extended PEG units (e.g., Fmoc-AEEEA) offer fine- tuned hydrophilicity and spacing. 8. Comparison with Other Linkers • Fmoc-Gly-OH: Simpler and cheaper but lacks hydrophilicity and flexibility. • Fmoc-PEGn-OH: Longer PEG chains (n = 4–12) provide greater solubility but increase size and cost. • Fmoc-Ahx-OH: Hydrophobic 6-aminohexanoic acid offers spacing but not solubility enhancement. • Beta-Alanine: Shorter and less hydrophilic, suitable for minimal spacing. Fmoc-AEEA balances size, solubility and ease of use, making it a preferred linker for many applications. Fmoc-AEEA is a highly effective hydrophilic spacer in peptide synthesis and bioconjugation, offering flexibility, solubility, and seamless integration into Fmoc SPPS. Its role in improving peptide therapeutics, diagnostics, and biomaterials highlights its versatility and practical value. While cost and lack of rigidity pose minor limitations, ongoing advancements in automation, green chemistry and conjugation strategies continue to expand its utility. Fmoc-AEEA is a critical reagent in peptide chemistry, driving innovation in biomolecular design and therapeutic development. References 1. Overview of PEG Linkers 2. Overview of Polyethylene Glycol (PEG) 3. Polyethylene Glycol 4. PEGylation 5. PEGylation – an overview 6. PEGylation, successful approach to drug delivery 7. Peptide Synthesis 8. Overview of Solid Phase Synthesis 9. Fmoc Solid Phase Peptide Synthesis 10. Boc Solid Phase Peptide Synthesis |
|
Fmoc-NH-PEG2-CH2COOH
For Research & Development use only. Not for testing and/or use on humans.