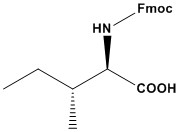
| Synonym: | N-α-Fmoc-D-isoleucine |
| CAS #: | 143688-83-9 |
| Molecular Formula: | C21H23NO4 |
| Molecular Weight: | 353.4 |
| Fmoc-D-Ile-OH (N-(9-Fluorenylmethoxycarbonyl)-D-isoleucine) is a chiral, non-natural amino acid derivative widely used in solid-phase peptide synthesis (SPPS). It belongs to the class of Fmoc- protected amino acids, where the 9-fluorenylmethoxycarbonyl (Fmoc) group serves as a temporary protecting group for the α-amino functionality, while the carboxylic acid remains free for peptide bond formation. The D-configuration of isoleucine makes it valuable for synthesizing peptides with specific stereochemical requirements, such as antimicrobial peptides, enzyme inhibitors, and peptidomimetics. 1. Applications of Fmoc-D-Ile-OH 1.1 Peptide and Protein Engineering Fmoc-D-Ile-OH is extensively used in the synthesis of peptides containing D-amino acids, which are essential for: • Enhancing proteolytic resistance – D-amino acids increase peptide stability against enzymatic degradation in biological systems. • Modulating secondary structures – Incorporating D-isoleucine can disrupt α-helices and β-sheets, impacting peptide folding and function. • Improving bioavailability – Peptides with D-amino acids often exhibit increased half-life and better pharmacokinetic properties. 1.2 Drug Development & Peptide Therapeutics • Antimicrobial Peptides (AMPs): Many AMPs contain D-amino acids to improve stability and enhance antimicrobial activity by preventing degradation by proteases. • Cancer Research: Modified peptides with D-isoleucine have been studied for their potential anticancer activity by enhancing receptor binding and metabolic stability. • Neuropeptides & Hormone Analogs: Peptide drugs incorporating Fmoc-D-Ile-OH can improve binding affinity and receptor selectivity for therapeutic targets. 1.3 Structural Studies & Molecular Biology • Peptide Conformational Studies: Fmoc-D-Ile-OH is used in research focusing on how D- amino acids influence peptide folding and interaction. • Chirality & Enzyme Selectivity Studies: The inclusion of D-amino acids helps researchers understand enzyme specificity, protein-ligand interactions, and structural modifications. 2. Fmoc-D-Ile-OH in Solid-Phase Peptide Synthesis (SPPS) 2.1 Fmoc Strategy in Peptide Synthesis Fmoc-D-Ile-OH follows the Fmoc/t-Bu strategy, where: • The Fmoc group protects the α-amino group and is removed using mild bases (e.g., 20% piperidine in DMF). • The carboxyl group is activated (e.g., with HBTU/HOBt or DIC/DMAP) for efficient peptide bond formation. • The side-chain functional groups (if applicable) are protected with t-Bu-based protecting groups to ensure selective deprotection. 2.2 Coupling Efficiency & Challenges • High Coupling Efficiency: The incorporation of D-Ile proceeds with high yield, particularly when using efficient coupling reagents (e.g., HATU, DIC). • Avoiding Racemization: Unlike L-amino acids, D-amino acids inherently prevent racemization issues, ensuring higher purity peptides. • Solubility Considerations: Due to its hydrophobic nature, special solvent selection (DMF, DMSO, NMP) may be required for optimal solubilization. 3. Comparison with Other Derivatives • Fmoc-L-Ile-OH: Cheaper and more abundant but lacks D-isoleucine’s stability and structural benefits. • Boc-D-Ile-OH: Used in Boc SPPS with harsher cleavage (HF); less common due to Fmoc’s milder conditions. • Fmoc-D-Val-OH: Similar branched-chain D-amino acid but with a smaller isopropyl side chain, offering less hydrophobicity. • Unprotected D-Ile: Unsuitable for SPPS due to lack of α-amino protection. Fmoc-D-Ile-OH’s base-labile protection and compatibility with TFA cleavage make it a standout for D-amino acid incorporation. Fmoc-D-Ile-OH is a versatile, high-value amino acid derivative essential for advanced peptide synthesis. Its ability to confer protease resistance, structural diversity, and enhanced stability makes it indispensable in peptide therapeutics, foldamers, and biochemical research. Proper handling, optimized coupling strategies, and awareness of its challenges ensure successful applications in synthetic peptide chemistry. References 1. D-peptide 2. D-peptide Protease Resistance 3. D-Peptide and D-Protein Technology: Recent Advances, Challenges, and Opportunities 4. D-Amino Acids and D-Amino Acid-Containing Peptides: Potential Disease Biomarkers and Therapeutic Targets? 5. Inspiration from the mirror: D-amino acid containing peptides in biomedical approaches 6. D-Amino Acids Modulate the Cellular Response of Enzymatic-Instructed Supramolecular Nanofibers of Small Peptides 7. Method to generate highly stable D-amino acid analogs of bioactive helical peptides using a mirror image of the entire PDB 8. Peptide Synthesis 9. Overview of Solid Phase Synthesis 10. Fmoc Solid Phase Peptide Synthesis 11. Boc Solid Phase Peptide Synthesis 12. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences |
|
Fmoc-D-Ile-OH
For Research & Development use only. Not for testing and/or use on humans.