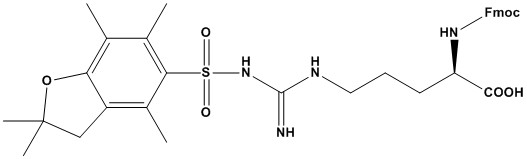
| Synonym: | N-α-Fmoc-N-g-(2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl)-D-arginine |
| CAS #: | 187618-60-6 |
| Molecular Formula: | C34H40N4O7S |
| Molecular Weight: | 648.8 |
| Fmoc-D-Arg(Pbf)-OH is a highly specialized and essential building block in modern peptide synthesis, particularly within the Fmoc/tBu solid-phase peptide synthesis (SPPS) strategy. It is the protected form of the D-enantiomer of Arginine, a crucial basic and polar amino acid. The use of D-amino acids, which are non-natural, is common in medicinal chemistry to create peptides with enhanced stability against enzymatic degradation, leading to improved pharmacokinetic properties. This review will delve into the structural features, synthetic role, and practical considerations of this important chemical compound. 1. Chemical Structure and Properties Its structure is composed of three key parts: • Fmoc Group (Nα-9-fluorenylmethyloxycarbonyl): This is the Nα-amino protecting group. The Fmoc group is renowned for its stability under acidic conditions but is readily removed by a weak base, typically a solution of piperidine. This base-labile characteristic allows for selective deprotection of the N-terminus at each coupling step in an Fmoc/tBu-based SPPS. • D-Arginine Residue: This is the D-enantiomer of the natural amino acid Arginine. D-amino acids are stereoisomers of L-amino acids. Their incorporation into a peptide chain can dramatically alter the peptide’s secondary structure and its resistance to proteases, which are highly specific for L-amino acids. • Pbf Group (2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl): This is the side-chain protecting group for the guanidino group of Arginine. The guanidinium side chain of Arginine is highly basic and reactive, making it necessary to protect it during synthesis to prevent unwanted side reactions. The Pbf group is the gold standard for this purpose. It is highly acid-labile and can be efficiently removed in the final cleavage step using trifluoroacetic acid (TFA), which is also used to cleave the peptide from the resin. 2. Role in Solid-Phase Peptide Synthesis (SPPS) In the Fmoc/tBu strategy of SPPS, a peptide chain is built one amino acid at a time on a solid resin support. The process for incorporating Fmoc-D-Arg(Pbf)-OH involves the following steps: • Fmoc Deprotection: The Fmoc group of the previous amino acid on the resin is removed using a mild base (e.g., 20% piperidine in DMF), exposing the free Nα-amino group. • Coupling: Fmoc-D-Arg(Pbf)-OH, activated by a coupling reagent (e.g., HATU, HOBt/DIC), is added to the resin. The activated carboxyl group of Fmoc-D-Arg(Pbf)-OH reacts with the free Nα-amino group on the resin, forming a new peptide bond. • Washing: The resin is thoroughly washed to remove excess reagents and byproducts. This cycle is repeated for each amino acid in the sequence. The Pbf protecting group on the Arginine side chain remains intact throughout the synthesis, unaffected by the piperidine used for Fmoc deprotection. 3. Advantages of Fmoc-D-Arg(Pbf)-OH • Orthogonal Protecting Group Strategy: The Fmoc (base-labile) and Pbf (acid-labile) groups are orthogonally protected. This means that one group can be removed without affecting the other, allowing for the precise, stepwise construction of the peptide chain. • Preventing Side Reactions: The Pbf group effectively suppresses the high basicity and nucleophilicity of the Arginine side chain, preventing undesirable side reactions like guanidino group acylation, which could lead to truncated or incorrect peptide sequences. • Enhanced Cleavage: The Pbf group is highly sensitive to acid, making it easier to cleave in the final step compared to older protecting groups like tosyl (Tos), which required harsher conditions and could cause side reactions. • Biologically Active Peptides: The use of the D-enantiomer allows for the synthesis of peptides that are resistant to proteases, enzymes that typically cleave L-amino acid-based peptides. This is a critical feature for developing peptide-based therapeutics with a longer half-life in biological systems. 4. Handling and Storage Fmoc-D-Arg(Pbf)-OH is a stable compound when stored correctly. • Physical Form: It is typically a white or off-white powder. • Stability: It is stable under normal laboratory conditions but is sensitive to prolonged exposure to light, heat, and moisture. • Storage: It should be stored in a cool, dark, and dry place, preferably in a freezer or refrigerator (e.g., -20°C). The container should be tightly sealed to prevent moisture absorption. 5. Deprotection and Final Cleavage After the entire peptide chain has been assembled on the resin, the final step is to cleave the peptide from the solid support and remove all the remaining acid-labile protecting groups, including the Pbf group on Arginine. • Final Cleavage Cocktail: This is typically performed using a strong acid cocktail, with trifluoroacetic acid (TFA) as the primary component. The cocktail also includes scavengers (e.g., water, triisopropylsilane (TIS), and/or thioanisole) to trap carbocations generated during the cleavage process, which prevents them from reacting with sensitive residues in the peptide. • Pbf Cleavage: The Pbf group is cleaved effectively and cleanly by the TFA in the cocktail, restoring the free guanidino side chain of Arginine. 6. Applications Fmoc-D-Arg(Pbf)-OH is a critical component in a variety of research and development fields: • Medicinal Chemistry: For the synthesis of therapeutic peptides that need to be orally bioavailable or have an extended half-life in the bloodstream. • Drug Discovery: Creating peptide libraries with D-amino acids to screen for novel compounds with improved pharmacological properties. • Biochemical Research: Studying the role of D-amino acids in natural systems and creating stable peptide probes for structural biology. • Enzyme Inhibitor Development: Synthesizing peptides that act as highly specific and stable inhibitors of various proteases. Fmoc-D-Arg(Pbf)-OH is a highly sophisticated and indispensable reagent for modern peptide synthesis. Its carefully designed protecting groups allow for a robust and efficient SPPS strategy, while the incorporation of the D-amino acid provides a powerful tool for creating enzymatically stable and pharmacologically valuable peptides. Its ease of handling and reliable deprotection make it a workhorse in laboratories focused on peptide chemistry, from academic research to pharmaceutical development. References: 1. Peptide Synthesis 2. Fmoc Solid Phase Peptide Synthesis 3. Boc Solid Phase Peptide Synthesis 4. Chan, W. C., & White, P. D. (2000). Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford University Press. 5. Albericio, F., & El-Faham, A. (2009). Choosing the Right Coupling Reagent for Peptides: A Twenty-Five-Year Journey. Org. Process Res. Dev., 13, 6–13. 6. Goodman, M., & Felix, A. (1995). Synthetic Peptides: A User’s Guide. W.H. Freeman. 7. Bray, B. L. (2003). Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov., 2, 587–593. |
|
Fmoc-D-Arg(Pbf)-OH
For Research & Development use only. Not for testing and/or use on humans.