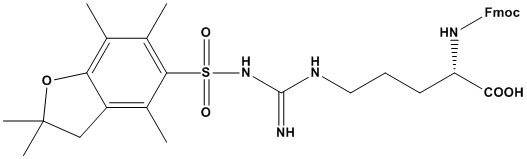
| Synonym: | N-α-Fmoc-N-g-(2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl)-L-arginine |
| CAS #: | 154445-77-9 |
| Molecular Formula: | C34H40N4O7S |
| Molecular Weight: | 648.8 |
| Fmoc-Arg(Pbf)-OH is a protected derivative of the amino acid arginine, widely used in solid-phase peptide synthesis (SPPS), particularly in Fmoc (fluorenylmethyloxycarbonyl) chemistry. It is essential for incorporating arginine into peptide sequences while preventing side reactions during synthesis. Below is a detailed review of its properties, applications, and considerations: 1. Properties of Fmoc-Arg(Pbf)-OH (1). Chemical Structure: Fmoc-Arg(Pbf)-OH consists of three main components: Fmoc group: A protecting group for the α-amino group, removable under basic conditions (e.g., piperidine). Pbf group (2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl): A protecting group for the guanidine side chain of arginine, which is acid-labile and removed during the final cleavage step. Arginine backbone: The amino acid core, which includes a carboxylic acid group for coupling to the growing peptide chain. (2). Solubility: Fmoc-Arg(Pbf)-OH is soluble in polar organic solvents such as DMF (dimethylformamide) and DMSO (dimethyl sulfoxide), making it suitable for SPPS. (3). Stability: The Fmoc group is stable under acidic conditions but can be removed under basic conditions (e.g., 20% piperidine in DMF). The Pbf group is stable under basic conditions but can be cleaved with strong acids, such as trifluoroacetic acid (TFA), during the final deprotection step. (4). Side Chain Protection: The Pbf group is specifically designed to protect the highly reactive guanidine group of arginine, preventing side reactions such as cyclization or branching during peptide synthesis. 2. Applications of Fmoc-Arg(Pbf)-OH (1). Peptide Synthesis: Fmoc-Arg(Pbf)-OH is used to incorporate arginine into peptide sequences during Fmoc-based SPPS. Arginine is a common amino acid in peptides due to its positive charge and role in protein-protein interactions. (2). Therapeutic Peptides: Arginine-rich peptides are often used in drug development, particularly for cell-penetrating peptides (CPPs) and antimicrobial peptides. Fmoc-Arg(Pbf)-OH ensures the correct incorporation of arginine without side reactions. (3). Bioconjugation: The protected arginine can be used in the synthesis of peptides for bioconjugation, where the guanidine group may play a role in binding or solubility. (4). Research and Development: Fmoc-Arg(Pbf)-OH is widely used in academic and industrial research for synthesizing peptides for structural, functional, and biochemical studies. 3. Advantages (1). Efficient Protection: The Pbf group effectively protects the guanidine side chain, preventing side reactions and ensuring high yields during peptide synthesis. (2). Compatibility: Fmoc-Arg(Pbf)-OH is compatible with standard Fmoc-SPPS protocols, including automated synthesizers. (3). High Purity: Commercially available Fmoc-Arg(Pbf)-OH is typically of high purity, ensuring reliable and reproducible results. (4). Selective Deprotection: The Pbf group can be selectively removed during the final cleavage step without affecting other protecting groups that may be present in the peptide. 4. Limitations (1). Deprotection Conditions: The Pbf group requires strong acids (e.g., TFA) for removal, which may not be compatible with acid-sensitive peptides or functional groups. (2). Steric Hindrance: The bulky Pbf group can sometimes cause steric hindrance during coupling reactions, particularly in sequences with multiple arginine residues. 5. Recent Advancements (1). Improved Coupling: Microwave-assisted SPPS enhances Fmoc-Arg(Pbf)-OH incorporation, reducing reaction times and improving yields in arginine-rich sequences. (2). Green Chemistry: Alternatives to DMF (e.g., 2-MeTHF) and reduced TFA volumes in cleavage cocktails improve sustainability. (3). Automation: Automated synthesizers (e.g., Liberty Blue) optimize Fmoc-Arg(Pbf)-OH handling, minimizing manual errors. (4). Custom Derivatives: Variants like Fmoc-D-Arg(Pbf)-OH expand applications to D-amino acid peptides for enhanced stability. (5). Analytical Advances: High-resolution MS and NMR refine quality control, ensuring ultra-high purity for pharmaceutical use. 6. Comparison with Other Arginine Derivatives (1). Fmoc-Arg(Mtr)-OH: Uses the Mtr (4-methoxy-2,3,6-trimethylbenzenesulfonyl) protecting group, which is also acid-labile but less commonly used due to lower stability. (2). Fmoc-Arg(Pmc)-OH: Uses the Pmc (2,2,5,7,8-pentamethylchroman-6-sulfonyl) protecting group, which is similar to Pbf but less sterically hindered. (3). Fmoc-Arg(Boc)2-OH: Uses Boc (tert-butoxycarbonyl) groups for side chain protection, which require harsher deprotection conditions. Among these, Fmoc-Arg(Pbf)-OH is the most widely used due to its balance of stability, ease of deprotection, and compatibility with SPPS. Fmoc-Arg(Pbf)-OH is a critical reagent for incorporating arginine into peptides during Fmoc-based SPPS. Its effective protection of the guanidine side chain, compatibility with standard protocols, and high purity make it a preferred choice for peptide synthesis. However, its cost, steric hindrance, and requirement for strong acid deprotection should be considered when planning experiments. Overall, Fmoc-Arg(Pbf)-OH is an indispensable tool for synthesizing arginine- containing peptides in both research and therapeutic applications. References: 1. Peptide Synthesis 2. Overview of Solid Phase Synthesis 3. Fmoc Solid Phase Peptide Synthesis 4. Boc Solid Phase Peptide Synthesis 5. Solid-phase peptide synthesis: from standard procedures to the synthesis of difficult sequences |
|