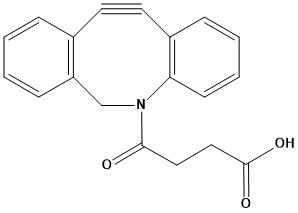
| Synonym: | Dibenzocyclooctyne acid |
| CAS #: | 1353016-70-2 |
| Molecular Formula: | C19H15NO3 |
| Molecular Weight: | 305.3 |
| DBCO-Acid, also known as Dibenzocyclooctyne-acid or DBCO-COOH, is a crucial reagent in modern chemical biology and materials science, primarily known for its role in copper-free click chemistry. It combines a highly strained alkyne (dibenzocyclooctyne, DBCO) with a carboxylic acid functional group, making it a versatile building block for diverse bioconjugation and material functionalization applications. 1. Chemical Structure and Properties • Common Synonyms: DBCO-COOH, DBCO-acid, Dibenzocyclooctyne-acid, Azadibenzocyclooctyne acid. • CAS Number: 1353016-70-2 • Structure: The core of DBCO-Acid is the dibenzocyclooctyne (DBCO) moiety, which is a bicyclic ring system containing a strained alkyne. This strain is key to its reactivity in copper-free click chemistry. Attached to this is an amide linker connected to a short alkyl chain terminating in a carboxylic acid (-COOH) group. • Solubility: Soluble in common organic solvents like dimethyl sulfoxide (DMSO) and dimethyl formamide (DMF). Sparingly soluble in water, but typically used in aqueous buffers with a small percentage of organic co-solvent or after conversion to a more water-soluble derivative. • Stability: Generally stable as a solid at -20°C. It can be light-sensitive, moisture-sensitive, and heat-sensitive, requiring appropriate storage conditions. Solutions should be prepared freshly or stored in aliquots to maintain reactivity. 2. Principle of Reactivity: Copper-Free Click Chemistry The defining feature of DBCO-Acid is its participation in Strain-Promoted Alkyne-Azide Cycloaddition (SPAAC), often referred to as “copper-free click chemistry.” 2.1 Mechanism The inherent ring strain within the cyclooctyne structure of DBCO activates the alkyne, allowing it to react spontaneously with an azide group without the need for a copper(I) catalyst. This reaction forms a stable 1,2,3-triazole linkage. 2.2 Advantages over CuAAC • Biocompatibility: Eliminates the need for cytotoxic copper catalysts, making it ideal for labeling biomolecules in live cells, in vivo, or in sensitive biological systems where copper ions could cause denaturation or toxicity. • Orthogonality: The reaction is highly specific and does not typically react with other common biological functional groups like amines, thiols, or hydroxyls, which are prevalent in proteins and other biomolecules. This allows for precise modification without unwanted side reactions. • Fast Kinetics: While generally slower than CuAAC, DBCO-based SPAAC reactions are sufficiently fast for many biological applications, often completing within hours at room temperature. • Versatility: Can be used in various solvents, including aqueous buffers. 3. Applications DBCO-Acid’s unique combination of a reactive cyclooctyne and a versatile carboxylic acid group makes it a highly valuable tool across various scientific disciplines: 3.1 Bioconjugation • Protein & Peptide Labeling: The carboxylic acid group can be activated (e.g., using EDC/NHS or HATU) to form an activated ester that reacts readily with primary amines (lysine residues, N-termini) on proteins and peptides. This allows for the site-specific or random labeling of biomolecules with the DBCO handle. • Nucleic Acid Modification: Can be conjugated to amine-modified oligonucleotides for creating DNA/RNA probes or for immobilizing nucleic acids onto surfaces. • Lipid & Carbohydrate Functionalization: Used to modify these biomolecules for studies on cell membranes, glycoengineering, and targeted delivery. • Antibody-Drug Conjugates (ADCs): Serves as a linker component in the synthesis of ADCs. For example, DBCO-Acid can be linked to a drug payload, and then this DBCO-drug conjugate can be attached to an azide-modified antibody. • Cell Surface Labeling: Enables the tagging of specific biomolecules on the surface of live cells without causing toxicity. This is crucial for visualizing cellular processes, tracking cell migration, or engineering cell surfaces. 3.2 Materials Science and Nanotechnology • Surface Functionalization: Used to immobilize azide-containing biomolecules or polymers onto surfaces of biosensors, microarrays, nanoparticles, and medical implants. This creates functionalized surfaces with specific biological properties or reactivity. • Polymer Synthesis & Modification: Can be incorporated into polymers to create clickable materials for hydrogels, drug delivery vehicles, and other advanced materials. • Nanoparticle Conjugation: Enables the precise attachment of biomolecules to nanoparticles for targeted delivery, imaging, or sensing applications. 3.3 Drug Discovery and Development • Prodrug Strategies: Can be incorporated into prodrugs where the click reaction triggers drug release at a specific site or time. • Fragment-Based Drug Discovery: Used to connect fragments or explore protein-ligand interactions using click chemistry. • Molecular Probes: Synthesizing probes for target identification, validation, and elucidation of biological pathways. 3.4 Diagnostics • Biosensor Development: Fabrication of highly selective biosensors by immobilizing biomolecules via click chemistry. • Immunoassays: Developing new generations of ELISA or other immunoassay platforms with improved efficiency and specificity. 4. Advantages • Copper-Free Reactivity: Its most significant advantage, allowing safe and efficient bioconjugation in biological systems. • High Specificity: Excellent orthogonality with common biological functional groups, minimizing off-target reactions. • Stable Linkage: Forms a stable triazole bond that is resistant to enzymatic degradation and remains intact under physiological conditions. • Versatile Carboxylic Acid: The -COOH group allows for diverse downstream modifications, including amide coupling with amines, esterification with alcohols, or further activation for other conjugation chemistries. • Minimal Spacer: Adds minimal bulk to the modified molecule, which can be important for maintaining biological activity. 5. Disadvantages and Considerations • Reaction Rate: While faster than some other copper-free methods, SPAAC is generally slower than CuAAC, which might require longer incubation times for certain applications. • Hydrophobicity: The dibenzocyclooctyne core can be somewhat hydrophobic, which might limit its solubility or lead to aggregation issues when conjugating to highly polar or water-soluble biomolecules. This is often addressed by incorporating hydrophilic spacers (like PEG) in DBCO-containing linkers. • Storage: Requires careful storage conditions to maintain reactivity, especially as a solution. DBCO-Acid is an indispensable tool in the burgeoning field of bioorthogonal chemistry. Its ability to undergo rapid and highly specific copper-free click reactions with azides, combined with the versatility of its carboxylic acid handle, has revolutionized how researchers label and functionalize biomolecules and materials. From advancing targeted drug delivery and molecular imaging to facilitating the creation of novel biosensors and polymeric scaffolds, DBCO-Acid continues to empower innovative discoveries at the interface of chemistry and biology, enabling studies in complex biological environments that were previously challenging or impossible. As demand for biocompatible and precise conjugation methods grows, DBCO-Acid remains at the forefront of chemical toolkit development. References 1. Overview of Click Chemistry 2. Click chemistry 3. Copper-free click chemistry 4. Introduction: Click Chemistry 5. Click Chemistry Azide-Alkyne Cycloaddition |
|
DBCO-Acid
For Research & Development use only. Not for testing and/or use on humans.