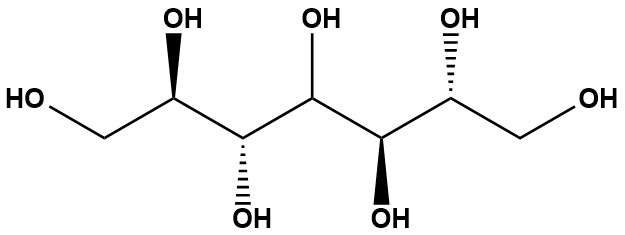
| Synonym: | Volemitol; D-Glycero-D-talo-heptitol; D-Glycero-D-manno-heptitol |
| CAS #: | 488-38-0 |
| Molecular Formula: | C7H16O7 |
| Molecular Weight: | 212.2 |
| D-Volemitol (CAS #: 488-38-0), also known as D-glycero-D-manno-heptitol or α-sedoheptitol, is a naturally occurring seven-carbon sugar alcohol (heptitol) with the chemical formula C7H16O7. It is widely distributed across various organisms, including plants, fungi, red algae, mosses, and lichens, and has been identified in bacterial lipopolysaccharides, such as those from Escherichia coli. First isolated in 1889 by the French scientist Émile Bourquelot from the mushroom Lactarius volemus, D-Volemitol has garnered attention for its physiological roles in certain plants, particularly in the genus Primula, as well as its potential applications as a natural sweetener and pharmaceutical excipient. 1. Natural Occurrence and Distribution D-Volemitol is a polyol found in diverse biological systems, reflecting its evolutionary significance and functional versatility. It is present in: • Plants: Notably abundant in certain species of the genus Primula (e.g., Primula × polyantha, Primula officinalis), where it serves as a major nonstructural carbohydrate. Concentrations in Primula leaves can reach up to 50 mg/g fresh weight (approximately 25% of dry weight), making it the dominant soluble carbohydrate in these species, followed by sedoheptulose (36 mg/g fresh weight) and sucrose (4 mg/g fresh weight). It is also found in low amounts in avocado seeds and is absent in some Primula species from sections such as Auriculastrum and Aleuritia. • Fungi: Initially isolated from Lactarius volemus (now Lactifluus volemus), a mushroom prevalent in temperate regions of the Northern Hemisphere, D-Volemitol is a key metabolite in certain fungal species. • Algae: Red algae (Rhodophytes), such as Pelvetia canaliculata, synthesize D-Volemitol, potentially via a pathway involving sedoheptulose 7-phosphate and NADH-dependent reductase. • Mosses and Lichens: D-Volemitol’s presence in these organisms suggests a role in stress tolerance, particularly in extreme environmental conditions, due to its osmotic and protective properties. • Bacteria: It is a component of lipopolysaccharides in E. coli, indicating a role in bacterial cell wall structure. The restricted distribution of D-Volemitol within specific Primula sections (e.g., subgenus Primula) highlights its chemotaxonomic significance, potentially serving as a marker for phylogenetic relationships within the genus. 2. Chemical and Physical Properties D-Volemitol is a hepta-hydroxy alcohol with the IUPAC name (2R,3R,5R,6R)-heptane-1,2,3,4,5,6,7-heptol. Its key properties include: • Molecular Formula: C7H16O7 • Molecular Weight: 212.20 g/mol • Appearance: White crystalline substance • Melting Point: 152–153°C • Solubility: Highly soluble in water, contributing to its role as an osmotic agent • Taste: Slightly sweet, less intense than xylitol but with favorable consumer acceptance due to its low caloric content Structurally, D-Volemitol is a reduction product of the ketose sugar sedoheptulose, distinguished from other heptitols (e.g., perseitol) through techniques like high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) or gas chromatography-mass spectrometry (GC-MS). Its Fischer projection reveals a stereospecific arrangement, with synonyms including D-glycero-D-talo-heptitol and β-mannoheptitol. 3. Chemical Reactions D-Volemitol undergoes several chemical reactions relevant to its biological and industrial applications: • Oxidation: Using strong oxidizing agents like potassium permanganate or nitric acid, D-Volemitol can be oxidized to form heptonic acids. • Reduction: It is synthesized in plants via the reduction of sedoheptulose by a NADPH-dependent sedoheptulose reductase, a novel enzyme identified in Primula species. • Substitution: Hydroxyl groups on D-Volemitol can be substituted to yield derivatives with varied functional groups, potentially useful in chemical synthesis or drug formulation. 4. Biosynthesis In Primula species, D-Volemitol biosynthesis is mediated by a unique NADPH-dependent ketose reductase, tentatively named sedoheptulose reductase. This enzyme reduces sedoheptulose (D-altro-2-heptulose) to D-Volemitol with high substrate specificity. Key characteristics of sedoheptulose reductase include: • pH Optimum: 7.0–8.0 • Substrate Affinity: Apparent Km of 21 mM for sedoheptulose and 0.4 mM for NADPH • Specificity: High specificity for sedoheptulose, distinguishing it from other reductases that typically use aldose sugars or phosphorylated substrates. In brown algae like Pelvetia canaliculata, an alternative pathway involves sedoheptulose 7-phosphate and an NADH-dependent reductase, producing D-Volemitol via volemitol 1-phosphate. These pathways highlight the diversity of D-Volemitol biosynthesis across organisms, with implications for its physiological roles. 5. Physiological Roles in Plants D-Volemitol serves multiple critical functions in Primula species, particularly in the horticultural hybrid Primula × polyantha: • Photosynthetic Product: Pulse-chase experiments with 14CO2 demonstrate that D-Volemitol is a major photosynthetic product, following sedoheptulose in the metabolic pathway. This suggests it is actively synthesized during photosynthesis, contributing to carbon assimilation. • Phloem Translocate: D-Volemitol is a prominent phloem-mobile carbohydrate, constituting approximately 24% (mol/mol) of phloem sap carbohydrates in Primula, second only to sucrose (63%). This mobility facilitates the transport of carbon and energy to sink organs like roots and developing leaves. • Storage Carbohydrate: High concentrations in leaves (up to 50 mg/g fresh weight) and presence in roots indicate D-Volemitol’s role as a storage compound, supporting plant growth and overwintering. Its accumulation in both aboveground and underground organs suggests potential as a cryoprotectant, enhancing freezing tolerance in temperate Primula species, though direct evidence is lacking. • Stress Tolerance: The presence of D-Volemitol in lichens and mosses, known for surviving extreme conditions, suggests a role in osmotic regulation and stress protection, potentially stabilizing cellular structures under desiccation or temperature stress. 6. Potential Applications D-Volemitol’s unique properties position it for various applications in food, pharmaceutical, and industrial contexts: 6.1 Food Industry • Sugar Substitute: As a low-calorie sweetener, D-Volemitol offers a less sweet but consumer-preferred taste profile compared to xylitol, suitable for diabetic-friendly or reduced-calorie products. • Texturizing Agent: Its moisture-retaining properties make it a candidate for improving the texture of food products, such as baked goods or confections. 6.2 Pharmaceutical Industry • Osmotic Diuretic: Structurally similar to mannitol, D-Volemitol may function as an osmotic diuretic for managing conditions like cerebral edema or elevated intraocular pressure, though clinical studies are needed. • Drug Delivery: Studies suggest D-Volemitol enhances the stability and solubility of active pharmaceutical ingredients (APIs), making it a potential excipient in oral medication formulations. 6.3 Biotechnology Its role in stress tolerance in lichens and mosses could inspire biomimetic applications, such as developing stress-resistant crops or biocompatible materials. 7. Analytical Methods Accurate identification and quantification of D-Volemitol require advanced analytical techniques due to its structural similarity to other heptitols: • HPAEC-PAD: High-performance anion-exchange chromatography with pulsed amperometric detection is used to quantify D-Volemitol in plant extracts, as seen in studies of Primula leaves. • GC-MS: Gas chromatography-mass spectrometry distinguishes D-Volemitol from related compounds like perseitol, aiding in precise chemical characterization. • Radiolabeling: 14CO2 pulse-chase experiments have elucidated D-Volemitol’s role in photosynthetic pathways, confirming its metabolic significance. 8. Critical Analysis and Future Directions While D-Volemitol’s physiological roles in Primula and other organisms are well-documented, several knowledge gaps remain: • Cryoprotectant Role: The hypothesis that D-Volemitol acts as a cryoprotectant in Primula species requires experimental validation, particularly through studies on freezing tolerance in transgenic plants with altered D-Volemitol levels. • Pharmaceutical Potential: Its osmotic and stabilizing properties warrant further investigation for drug delivery and therapeutic applications, with clinical trials needed to confirm efficacy and safety. • Biosynthetic Pathways: The diversity of D-Volemitol biosynthesis (e.g., NADPH- vs. NADH-dependent pathways) suggests potential for bioengineering enzymes like sedoheptulose reductase to enhance D-Volemitol production in industrial systems. • Ecological Significance: The role of D-Volemitol in stress tolerance in lichens and mosses could inform ecological studies on adaptation to extreme environments, potentially guiding conservation strategies. The limited sweetness of D-Volemitol compared to other sugar alcohols like xylitol may restrict its adoption as a sweetener, but its low caloric content and favorable taste profile offer niche opportunities in health-focused food products. Additionally, its restricted distribution in Primula species raises questions about its evolutionary origins and potential as a chemotaxonomic marker, meriting further genomic and metabolomic studies. D-Volemitol is a versatile seven-carbon sugar alcohol with significant physiological roles in Primula species, fungi, algae, mosses, and lichens. Its functions as a photosynthetic product, phloem translocate, and storage carbohydrate underscore its importance in plant metabolism, while its presence in stress-tolerant organisms suggests broader ecological roles. Chemically, D-Volemitol’s stability, solubility, and reactivity make it a promising candidate for food and pharmaceutical applications, particularly as a low-calorie sweetener and drug excipient. However, further research is needed to fully elucidate its cryoprotective potential, optimize its biosynthesis, and explore its industrial applications. As a naturally occurring polyol with unique properties, D-Volemitol represents an exciting frontier in biochemical and applied research. References: 1. Volemitol 2. Volemitol 3. Metabolism of D-Glycero-D-Manno-Heptitol, Volemitol, in Polyanthus. Discovery of a Novel Ketose Reductase |
|
D-Volemitol
For Research & Development use only. Not for testing and/or use on humans.