| CAS #: | 2061897-68-3 |
| Molecular Formula: | C34H38N4O5 |
| Molecular Weight: | 582.7 |
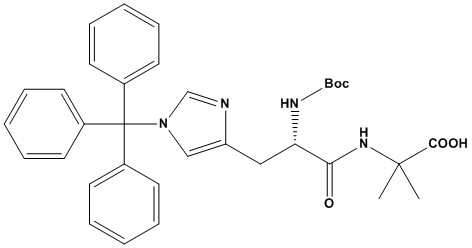
| Boc-His(Trt)-Aib-OH (CAS Number: 2061897-68-3) is a specialized dipeptide derivative widely utilized in peptide synthesis, particularly in solid-phase peptide synthesis (SPPS). This compound combines Nα-Boc-protected L-histidine with a trityl (Trt) protecting group on the imidazole side chain and α-aminoisobutyric acid (Aib), an unnatural amino acid. With a molecular formula of C 34 H 38 N 4 O 5 and a molecular weight of 582.69 g/mol, it is designed to facilitate the incorporation of histidine and Aib into peptide sequences, offering unique structural and functional properties 1. Chemical Structure and Properties Boc-His(Trt)-Aib-OH is chemically defined as (S)-2-(2-((tert-Butoxycarbonyl)amino)-3-(1-trityl-1H-imidazol-4-yl)propanamido)-2-methylpropanoic acid. Its structure includes: • Boc Protection: The tert-butoxycarbonyl (Boc) group protects the α-amino group of histidine, preventing unwanted side reactions during peptide coupling. It is removed under acidic conditions, typically with trifluoroacetic acid (TFA). • Trityl (Trt) Protection: The trityl group shields the τ-nitrogen of the histidine imidazole side chain, reducing racemization and side reactions like acylimidazole formation during coupling. The Trt group is also cleaved with TFA, making it compatible with Boc chemistry. • Aib Component: α-Aminoisobutyric acid, a non-proteinogenic amino acid with two methyl groups on the α-carbon, promotes helical conformations in peptides due to its steric hindrance, enhancing structural stability. • SMILES Notation: CC(C)(C)OC(=O)NC@@HC(=O)NC(C)(C)C(=O)O • Physical Properties: The compound is typically a solid with high purity (>95% by HPLC), stored at -20°C to maintain stability. It is soluble in common peptide synthesis solvents like DMF and NMP. These features make Boc-His(Trt)-Aib-OH a robust building block for constructing peptides with specific conformational and biological properties. 2. Applications in Peptide Synthesis Boc-His(Trt)-Aib-OH is primarily used in the biomedical and pharmaceutical industries for synthesizing peptides with tailored sequences. Its key applications include: 2.1 Peptide Drug Development • Targeted Therapies: The dipeptide is instrumental in creating peptides for diseases like Alzheimer’s, cancer, and metabolic disorders. The inclusion of Aib enhances peptide stability, making it suitable for designing long-acting therapeutics. • Semaglutide Synthesis: While Fmoc-His-Aib-OH is directly linked to semaglutide (a GLP-1 receptor agonist for type 2 diabetes), Boc-His(Trt)-Aib-OH serves as an alternative intermediate in similar peptide constructs, offering flexibility in Boc-based SPPS protocols. • Steroidogenesis and Hormone Regulation: Peptides incorporating histidine and Aib can interact with biological targets like mitochondrial transduceosomes, potentially influencing testosterone production or other hormonal pathways. 2.2 Structural Stability Studies • Aib’s α,α-disubstituted structure promotes helical conformations, reducing peptide degradation in vivo. This is critical for studying peptide interactions with biological macromolecules and designing resistant peptide architectures. • The Trt-protected histidine minimizes racemization, ensuring stereochemical integrity during synthesis, which is vital for studying structure-activity relationships. 2.3 Protein Engineering • Boc-His(Trt)-Aib-OH enables the design of proteins with enhanced stability or novel functions by incorporating non-natural amino acids. This is particularly useful in creating enzyme mimics or stabilized biologics. 2.4 DNA-Encoded Chemical Libraries (DECLs) • The compound’s compatibility with amide coupling conditions supports its use in DNA-encoded libraries, allowing rapid exploration of peptide chemical space for drug discovery. 3. Advantages of Boc-His(Trt)-Aib-OH The compound offers several benefits in peptide synthesis: • Racemization Suppression: The Trt group on histidine’s imidazole side chain effectively reduces racemization, a common issue with unprotected histidine, ensuring high enantiomeric purity. • Enhanced Peptide Stability: Aib’s steric properties induce helical structures, improving resistance to enzymatic degradation and extending peptide half-life in biological systems. • Compatibility with Boc Chemistry: The Boc and Trt groups are cleaved under similar acidic conditions (TFA), streamlining deprotection steps in SPPS. • Versatility: It supports the synthesis of peptides with both natural and unnatural amino acids, broadening the scope of peptide design for therapeutic and research purposes. 4. Limitations and Challenges Despite its strengths, Boc-His(Trt)-Aib-OH has some limitations: • Solubility Concerns: While soluble in DMF and NMP, its solubility may be lower than other Boc-protected amino acids, potentially complicating coupling reactions in certain solvent systems. • Deprotection Conditions: The use of TFA for Boc and Trt removal requires careful optimization to avoid side reactions, especially in peptides with sensitive residues. • Limited DNA-Compatible Deprotection: In DNA-encoded library synthesis, the Trt group’s deprotection is not fully compatible with DNA-friendly conditions, necessitating alternative protecting groups like 2-nitrobenzylsulfonyl for histidine. • Racemization Risk in Fmoc Chemistry: While the Trt group mitigates racemization in Boc chemistry, histidine racemization remains a concern in Fmoc-based SPPS, requiring careful selection of coupling reagents. 5. Practical Considerations in Use To maximize the utility of Boc-His(Trt)-Aib-OH, researchers should consider the following: • Storage and Handling: Store at -20°C in a dry environment to prevent degradation. Use anhydrous solvents to avoid hydrolysis of the Boc group. • Coupling Conditions: Employ efficient coupling reagents like HATU or DIC/HOBt to ensure complete amide bond formation, especially given Aib’s steric hindrance. Monitor for incomplete coupling, which can reduce yield. • Deprotection Optimization: Use TFA cocktails with scavengers (e.g., water, triisopropylsilane) to minimize side reactions during Boc and Trt removal. Adjust cleavage time based on peptide complexity. 6. Comparison with Alternatives Boc-His(Trt)-Aib-OH can be compared to related compounds like Fmoc-His-Aib-OH TFA and Boc-His(Bom)-OH: • Fmoc-His-Aib-OH TFA: Used in Fmoc-based SPPS, it is a direct intermediate for semaglutide synthesis. It lacks the Trt group, relying on TFA to handle side chain protection, which may increase racemization risk. It is less versatile in Boc chemistry but preferred for Fmoc protocols. • Boc-His(Bom)-OH: The Bom group on histidine’s τ-nitrogen is highly effective against racemization but is costlier and harder to prepare. It is less common in general synthesis but valuable for specific applications. • Boc-His(Trt)-OH: Lacks the Aib component, making it unsuitable for peptides requiring Aib’s structural benefits. It is simpler and cheaper but less specialized. Boc-His(Trt)-Aib-OH stands out for its dual functionality (histidine and Aib) and compatibility with Boc chemistry, making it ideal for complex peptide designs. Boc-His(Trt)-Aib-OH is a valuable protected dipeptide building block for peptide synthesis, particularly useful when incorporating histidine residues into conformationally constrained peptides. Its combination of the Boc strategy for N-terminal protection, trityl group for imidazole protection, and the conformationally restricting Aib residue makes it an excellent choice for researchers working with histidine-containing bioactive peptides. Despite challenges related to steric hindrance and potential aggregation, proper optimization of coupling conditions can lead to successful incorporation into target peptides. The compound offers an excellent balance of protection, reactivity, and stability for both solution and solid-phase peptide synthesis approaches. References 1. Peptide Synthesis 2. Fmoc Solid Phase Peptide Synthesis 3. Boc Solid Phase Peptide Synthesis 4. Chan, W.C. and White, P.D. (2000). Fmoc Solid Phase Peptide Synthesis: A Practical Approach. Oxford University Press. 5. Jaradat, D.M.M. (2018). “Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation.” Amino Acids, 50,39-68. 6. Jones, J. (2002). Amino Acid and Peptide Synthesis. Oxford University Press. 7. Góngora-Benítez, M., Tulla-Puche, J., and Albericio, F. (2013). “Handles for Fmoc Solid-Phase Synthesis of Protected Peptides.” ACS Comb. Sci., 15, 217-228. 8. Behrendt, R., White, P., and Offer, J. (2016). “Advances in Fmoc solid-phase peptide synthesis.” J. Pept. Sci., 22, 4-27. 9. Toniolo, C., Crisma, M., Formaggio, F., and Peggion, C. (2001). “Control of peptide conformation by the Thorpe-Ingold effect (Cα-tetrasubstitution).” Biopolymers, 60, 396-419. 10. Carpino, L.A. (1993). “1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive.” J. Am. Chem. Soc., 115, 4397-4398. 11. El-Faham, A. and Albericio, F. (2011). “Peptide Coupling Reagents, More than a Letter Soup.” Chem. Rev., 111, 6557-6602. |
|
Boc-His(Trt)-Aib-OH
For Research & Development use only. Not for testing and/or use on humans.