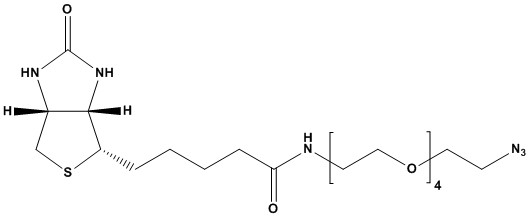
| Biotin-PEG4-azide is a sophisticated heterobifunctional linker molecule widely employed in various biochemical and biotechnological applications. It combines the high affinity of biotin for streptavidin/avidin with the solubility-enhancing and “stealth” properties of a polyethylene glycol (PEG) spacer, and the highly reactive azide functional group for click chemistry. This unique combination makes it an indispensable tool for site-specific labeling, purification, and functionalization of biomolecules and nanomaterials.
1. Chemical Structure and Properties
The molecule’s structure can be broken down into three key components:
1.1 Biotin (Vitamin H)
A small, water-soluble vitamin known for its exceptionally strong and specific non-covalent binding affinity to streptavidin and avidin proteins. This binding is one of the strongest known non-covalent interactions in biology, making biotin an ideal tag for affinity-based applications.
1.2 PEG4 (Polyethylene Glycol) Spacer
The tetraethylene glycol linker serves several crucial functions:
• Hydrophilicity: PEG chains are highly hydrophilic, increasing the aqueous solubility of the overall conjugate. This is particularly beneficial when conjugating hydrophobic molecules or working in aqueous biological environments.
• Reduced Steric Hindrance: The flexible and extended nature of the PEG spacer physically separates the biotin moiety from the molecule it is conjugated to. This helps minimize steric hindrance, ensuring optimal binding of biotin to streptavidin/avidin and reducing potential interference with the function of the target molecule.
• Reduced Non-Specific Binding (Stealth Effect): PEGylation is well-known to reduce non-specific interactions of conjugated molecules with biological components (e.g., cell surfaces, proteins), thus improving biocompatibility and reducing immunogenicity in some applications.
1.3 Azide (−N3) Group
This is a highly reactive functional group for various click chemistry reactions. It is relatively inert to most biological functional groups (bioorthogonal), allowing for selective conjugation without interfering with native biomolecules.
2. Applications
Biotin-PEG4-azide is a cornerstone reagent in chemical biology, enabling a wide array of applications due to its dual functionality:
2.1 Click Chemistry Biotinylation
• Copper-Catalyzed Azide-Alkyne Cycloaddition (CuAAC): The “gold standard” of click chemistry. The azide on Biotin-PEG4-azide reacts with terminal alkynes (e.g., propargyl-modified biomolecules) in the presence of a copper(I) catalyst to form a stable 1,2,3-triazole linkage. This is highly efficient and yields specific products.
• Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) / Copper-Free Click Chemistry: The azide reacts with strained cyclooctynes (e.g., DBCO, BCN) without the need for a toxic metal catalyst. This is crucial for applications in live cells or in vivo where copper toxicity is a concern.
2.2 Biomolecule Labeling and Functionalization
• Proteins and Antibodies: Labeling proteins or antibodies with biotin for detection in ELISA, Western blotting, immunohistochemistry, or for immobilization on streptavidin-coated surfaces.
• Nucleic Acids (DNA/RNA): Biotinylation of oligonucleotides for hybridization assays, pull-down experiments, or microarray applications.
• Lipids and Glycans: Metabolic labeling strategies often incorporate azide-modified sugars or lipids into cellular structures, which can then be “clicked” with Biotin-PEG4-azide for subsequent detection or isolation.
2.3 Drug Delivery Systems and Nanomedicine
• Targeted Drug Delivery: Biotinylated nanoparticles or liposomes can be prepared by conjugating Biotin-PEG4-azide to their surface. These can then be targeted to cells or tissues overexpressing streptavidin-tagged receptors, or used for affinity purification of drug carriers.
• PROTAC Synthesis: Some variants of Biotin-PEG-azide are used as linkers in the synthesis of Proteolysis Targeting Chimeras (PROTACs), where biotin serves as a purification tag or for functional studies.
2.4 Molecular Imaging and Diagnostics
• Fluorescent Labeling: While Biotin-PEG4-azide itself isn’t fluorescent, it can be used to conjugate a fluorescent tag to an alkyne-modified biomolecule, which is then detectable via the biotin-streptavidin interaction (e.g., using fluorescent streptavidin).
• Radiolabeling: In a similar vein, it can facilitate the incorporation of radioisotopes for PET or SPECT imaging, where the biotin provides a means for capture or targeting.
2.5 Protein Purification
• Affinity chromatography using streptavidin/avidin resins is a powerful method for purifying biotinylated proteins from complex mixtures. Biotin-PEG4-azide allows researchers to introduce this purification handle onto their target proteins.
2.6 Surface Immobilization
• Biotinylated molecules can be immobilized onto streptavidin-coated plates, beads, or biosensors for various assays, arrays, and diagnostic platforms.
3. Advantages
The widespread use of Biotin-PEG4-azide stems from several key advantages:
3.1 Bioorthogonality
The azide group is largely inert to endogenous biological functionalities, allowing for highly specific labeling reactions in complex biological systems (e.g., cell lysates, live cells, in vivo).
3.2 High Biotin-Streptavidin Affinity
This provides a robust and versatile handle for detection, purification, and immobilization applications.
3.3 PEG Spacer Benefits
• Enhanced Solubility: Improves handling and reaction efficiency in aqueous solutions.
• Reduced Non-Specific Interactions: Leads to cleaner results and less background noise in assays.
• Flexibility & Accessibility: The flexible PEG chain ensures the biotin moiety is accessible for binding, even when conjugated to large biomolecules or surfaces.
3.4 Versatility of Click Chemistry
Compatible with both copper-catalyzed (CuAAC) and copper-free (SPAAC) click reactions, offering flexibility based on the application’s sensitivity to copper.
3.5 Modular Design
Enables the facile combination of two distinct functionalities (biotin and azide) through a stable linker, providing a modular approach to construct complex bioconjugates.
3.6 Mild Reaction Conditions
Click chemistry reactions generally proceed efficiently under mild physiological conditions (neutral pH, room temperature), preserving the integrity and activity of sensitive biomolecules.
3.7 High Yields and Specificity
Click reactions are typically high-yielding and produce minimal byproducts, simplifying purification.
4. Limitations and Considerations
Despite its numerous advantages, some considerations are important when using Biotin-PEG4-azide:
• Copper Toxicity (for CuAAC): If CuAAC is used, the copper catalyst can be cytotoxic, limiting its use in live cell or in vivo applications. In such cases, copper-free click chemistry (SPAAC) with a strained alkyne partner is necessary.
• Regioisomeric Mixtures (for SPAAC): SPAAC reactions with unsymmetrical strained alkynes (like cyclooctynes) can sometimes yield a mixture of regioisomers, which might be a concern for applications requiring a single, defined product. However, for many labeling and detection purposes, this is not a significant issue.
• Steric Hindrance (even with PEG): While PEG reduces steric hindrance, very large or densely packed molecules might still experience some limitations in biotin-streptavidin binding efficiency.
• Detection Limits: While biotin-streptavidin is highly sensitive, the overall detection limit will depend on the concentration of the biotinylated target and the sensitivity of the detection method (e.g., fluorophore, enzyme conjugate).
Biotin-PEG4-azide is a powerful and essential tool in modern chemical biology and biotechnology. Its intelligent design, combining the robust biotin-streptavidin interaction with the bioorthogonal click chemistry handle and a solubility-enhancing, flexible PEG linker, enables researchers to precisely modify, track, purify, and target a vast array of biomolecules and materials. As research continues to push the boundaries of biological understanding and therapeutic development, reagents like Biotin-PEG4-azide will remain critical enablers for innovative discoveries.
References
1. Overview of PEG Linkers
2. Overview of Click Chemistry
3. Click chemistry
4. Copper-free click chemistry
5. Introduction: Click Chemistry
6. Click Chemistry Azide-Alkyne Cycloaddition
|