| Synonym: | N-4-pentynoyl-glucosamine-tetraacylated |
| CAS #: | 1361993-37-4 |
| Molecular Formula: | C19H25NO10 |
| Molecular Weight: | 427.4 |
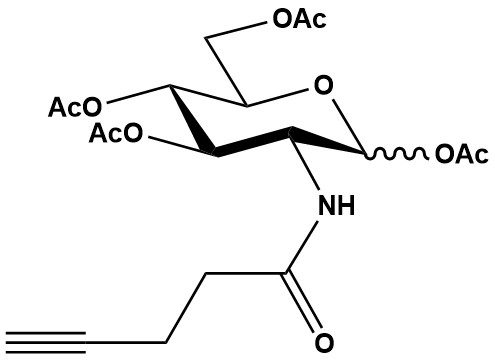
| Ac4GlcNAlk (N-4-pentynoyl-glucosamine-tetraacylated or N-Propargylglucosamine-tetraacetylated) is a widely used metabolic chemical reporter (MCR) in glycobiology and chemical biology. Its primary utility lies in studying O-linked β-N-acetylglucosamine (O-GlcNAc) modifications and, to some extent, N-linked glycosylation, by introducing an alkyne handle into cellular glycans. This alkyne tag then serves as a bioorthogonal handle for subsequent “click chemistry” reactions, particularly with azide-containing reporter molecules. 1. Mechanism of Action and Metabolic Labeling Ac4GlcNAlk operates via metabolic glycoengineering, a strategy that exploits the cell’s natural biosynthetic pathways: 1.1 Cell Permeability The peracetylated form (Ac4GlcNAlk) is lipophilic, allowing it to readily cross the hydrophobic cell membrane into the cytosol. 1.2 Intracellular Deacetylation Once inside the cell, intracellular carboxyesterases rapidly remove the acetyl groups, unmasking the free N-alkynylglucosamine (GlcNAlk). 1.3 Metabolic Incorporation: The deacetylated GlcNAlk enters the hexosamine biosynthetic pathway (HBP), acting as an analog of natural N-acetylglucosamine (GlcNAc). • O-GlcNAcylation: GlcNAlk is converted into its activated form, UDP-GlcNAlk, which can then serve as a substrate for O-GlcNAc transferase (OGT). OGT adds the GlcNAlk residue to serine and threonine residues of nuclear and cytoplasmic proteins, introducing an alkyne tag into O-GlcNAc-modified proteins. This is a primary application of Ac4GlcNAlk. • N-linked Glycosylation: GlcNAlk can also potentially be incorporated into N-linked glycans, which are synthesized in the ER and Golgi. However, the efficiency and specificity for N-glycans compared to O-GlcNAc can vary and might be influenced by factors like cell type and metabolic flux. 1.4 Bioorthogonal Ligation (Click Chemistry) The alkyne group on the metabolically incorporated glycoconjugates acts as a bioorthogonal handle for subsequent “click chemistry” reactions, typically with azide-containing reporter molecules: • Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC): This is the most common “click” reaction for alkynes. It is highly efficient and forms a stable triazole linkage. However, it requires a copper(I) catalyst, which can be cytotoxic, making it less suitable for live-cell or in vivo applications without careful optimization and use of copper-chelating ligands. • Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) or Copper-Free Click Chemistry: This reaction utilizes strained cyclooctynes (which contain a reactive alkyne group) to react with azides without the need for a copper catalyst. This makes Ac4GlcNAlk less compatible with SPAAC directly, as Ac4GlcNAlk itself contains the alkyne. Instead, Ac4GlcNAlk pairs with azide-tagged probes for CuAAC. For copper-free click, if you want to use GlcNAc analogs, you would typically use an azide-functionalized GlcNAc (like Ac4GlcNAz) and a cyclooctyne-containing probe. 2. Applications Ac4GlcNAlk is primarily used to study O-GlcNAcylation, a vital post-translational modification, and has several key applications: 2.1 Study of O-GlcNAcylation This is the most significant application. O-GlcNAcylation is a dynamic and reversible modification of thousands of nuclear, cytoplasmic, and mitochondrial proteins, regulating various cellular processes. Ac4GlcNAlk enables: • Visualization: Fluorescent labeling of O-GlcNAc-modified proteins for detection by fluorescence microscopy, flow cytometry, or in-gel fluorescence. • Proteomic Identification: Selective enrichment and identification of O-GlcNAc-modified proteins from complex cell lysates or tissues using biotinylated azide probes followed by streptavidin capture and mass spectrometry. • Functional Studies: Investigating the roles of O-GlcNAc in cell signaling, metabolism, gene expression, and its involvement in various diseases (e.g., cancer, diabetes, neurodegeneration). 2.2 Characterization of Glycosylation Changes Can be used to monitor changes in O-GlcNAcylation levels in response to various stimuli, cellular conditions, or disease progression. 2.3 Cell Surface and Secreted Protein Labeling (Less Common) While its primary focus is O-GlcNAc, if incorporated into N-linked glycans, it could potentially be used for cell surface labeling or secreted protein analysis, but the specificity for these applications is generally better with other MCRs (e.g., Ac4ManNAz for sialic acids, Ac4GalNAz for mucin-type O-glycans). 3. Advantages • Bioorthogonality: The alkyne group is largely absent from biological systems and is inert to most cellular components, leading to highly specific labeling. • Cell Permeability: The acetyl groups ensure efficient uptake by cells. • Versatility of Click Chemistry: The alkyne tag is amenable to CuAAC, which is robust and efficient for in vitro applications (e.g., cell lysates, fixed cells). • Reduced Metabolic Cross-Talk (Compared to Ac4GlcNAz): A notable advantage of Ac4GlcNAlk over Ac4GlcNAz (the azide version) for O-GlcNAc labeling is its reduced tendency for epimerization to GalNAlk by UDP-Glc/GalNAc 4-epimerase (GALE). This means that GlcNAlk is more specifically channeled into GlcNAc-dependent pathways, potentially offering a cleaner signal for O-GlcNAc modifications and reducing off-target labeling of other glycan types (e.g., mucin-type O-glycans). • Non-Radioactive: Provides a safe and convenient alternative to traditional radioisotope labeling. 4. Challenges and Limitations • Copper Toxicity (for CuAAC): The requirement for copper(I) catalyst in CuAAC is a major limitation for live-cell or in vivo applications due to copper’s cytotoxicity. While methods exist to mitigate this (e.g., using copper-chelating ligands, reducing reaction times), they add complexity. • Metabolic Efficiency: The efficiency of metabolic incorporation of GlcNAlk into O-GlcNAc can vary depending on the cell type, the concentration of the probe, and the activity of enzymes in the HBP. Metabolic bottlenecks can limit the biosynthesis of UDP-GlcNAlk. • Concentration Optimization: Proper optimization of the Ac4GlcNAlk concentration is crucial to achieve effective labeling without inducing metabolic stress or off-target effects. Typical concentrations for cell labeling are in the low to mid-micromolar range (e.g., 25-75 μM). • Potential for Non-Specific Binding (Less Common): While bioorthogonal, some non-specific interactions with highly reactive cellular components could theoretically occur, though this is generally minimal with proper experimental design. 5. Comparison with Ac4GlcNAz Ac4GlcNAlk and Ac4GlcNAz are both used to study O-GlcNAcylation. The main difference lies in their bioorthogonal handle (alkyne vs. azide) and the implications for click chemistry. • Handle Type: Ac4GlcNAlk provides an alkyne handle, typically reacted with an azide- containing probe via CuAAC. Ac4GlcNAz provides an azide handle, typically reacted with an alkyne-containing probe (either standard alkynes via CuAAC or cyclooctynes via SPAAC). • Specificity for O-GlcNAc: Critically, some studies suggest Ac4GlcNAlk can offer higher specificity for O-GlcNAc compared to Ac4GlcNAz, due to reduced epimerization of GlcNAlk to GalNAlk by GALE. This means less off-target labeling of mucin-type O-glycans (which typically initiate with GalNAc). Conversely, Ac4GalNAz (the azide version) has been shown to be surprisingly efficient at labeling O-GlcNAc due to its robust epimerization to GlcNAz. Therefore, the choice between alkyne- or azide-functionalized GlcNAc analogs depends on the specific research question, desired specificity, and the downstream click chemistry reaction planned. 6. Recent Research Advancements • Engineered Enzymes: Research focuses on engineering glycosyltransferases or enzymes in the HBP (e.g., engineered pyrophosphorylases like mut-AGX1) to enhance the metabolic conversion of Ac4GlcNAlk into UDP-GlcNAlk, thereby boosting labeling efficiency for O-GlcNAc modifications. • Combined Methodologies: Ac4GlcNAlk is increasingly used in conjunction with advanced mass spectrometry (MS)-based proteomics workflows to precisely identify and quantify O-GlcNAc modification sites on proteins. • GlycoRNA Discovery: Similar to azidosugars, Ac4GlcNAlk has also been implicated in the labeling of glycoRNA, expanding the understanding of glycosylation to a new class of biomolecules. Ac4GlcNAlk is a powerful chemical tool for investigating O-GlcNAcylation dynamics and identifying O-GlcNAc-modified proteins. Its alkyne handle facilitates robust click chemistry, and its potential for improved specificity regarding metabolic cross-talk offers a distinct advantage in certain applications over its azide counterpart, Ac4GlcNAz. References: 1. Ac4GlcNAlk 2. Chemical reporters for fluorescent detection and identification of O-GlcNAc-modified proteins reveal glycosylation of the ubiquitin ligase NEDD4-1 3. Overview of Click Chemistry |
|
Ac4GlcNAlk
For Research & Development use only. Not for testing and/or use on humans.
You may also like:
-
DBCO-PEG1-acid
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-β-D-galactopyranose
-
3,4,6-Tri-O-acetyl-2-azido-2-deoxy-D-galactopyranoside
-
Ac4ManNAz
-
Spermine(N3BBB)
-
2,3,4,6-Tetra-O-acetyl-β-D-galactopyranosyl Azide
-
2-Azidoethyl 2,3,4,6-Tetra-O-acetyl-β-D-galactopyranoside
-
2-[(Azidoacetyl)amino]-2-deoxy-D-galactopyranose
-
1,3,4,6-Tetra-O-acetyl-2-azido-2-deoxy-α-D-galactopyranose