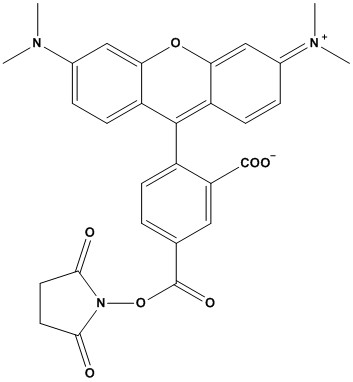
| Synonym: | 5-Carboxytetramethylrhodamine, succinimidyl ester |
| CAS #: | 150810-68-7 |
| Molecular Formula: | C29H25N3O7 |
| Molecular Weight: | 527.5 |
| 5-TAMRA, SE (5-Carboxytetramethylrhodamine, Succinimidyl Ester) is a widely used fluorescent labeling reagent in a variety of biological and chemical applications. As a single isomer of the tetramethylrhodamine (TAMRA) dye, it offers distinct advantages in specificity and reproducibility compared to mixed-isomer preparations. The “SE” (Succinimidyl Ester) functional group is key, as it provides a highly reactive site for covalent conjugation to biomolecules. 1. Chemical and Physical Properties • Chemical Name: 5-Carboxytetramethylrhodamine, Succinimidyl Ester • CAS Number: 150810-68-7 • Molecular Formula: C29H25N3O7 • Molecular Weight: 527.5 g/mol • Excitation/Emission Maxima: Approximately 546 nm (excitation) and 579 nm (emission), with some sources citing 553/575 nm • Extinction Coefficient: ~92,000 cm⁻¹M⁻¹ • Solubility: Soluble in anhydrous DMSO (dimethyl sulfoxide) or DMF (dimethylformamide); insoluble in aqueous solutions unless diluted immediately before use • Reactivity: Specifically targets primary amines (e.g., lysine residues in proteins or amine-modified oligonucleotides) to form stable covalent amide bonds at pH 7–9 • Stability: Stable in solid form when protected from light and moisture; susceptible to hydrolysis in aqueous solutions 5-TAMRA, SE is the single-isomer form of TAMRA, distinct from the mixed-isomer 5(6)-TAMRA, SE, which contains both 5- and 6-carboxy isomers. The single-isomer form offers better resolution in high-performance liquid chromatography (HPLC) purification, making it preferable for applications requiring high purity, such as peptide and protein labeling. 2. Applications 5-TAMRA, SE is a versatile fluorophore with applications across various fields of life sciences: 2.1 Protein and Peptide Labeling • bWidely used to conjugate with primary amines on proteins (e.g., antibodies) and peptides, forming fluorescent bioconjugates for immunochemistry, fluorescence microscopy, and flow cytometry. • Its bright, pH-insensitive fluorescence makes it ideal for visualizing protein localization and interactions in cells. 2.2 Nucleotide and Nucleic Acid Labeling • While 6-TAMRA, SE is more commonly used for nucleotide labeling, 5-TAMRA, SE is employed in specific cases for labeling amine-modified oligonucleotides, aiding in DNA sequencing and fluorescence-based assays. 2.3 Fluorescence Resonance Energy Transfer (FRET) • Frequently used as an acceptor dye in FRET studies, paired with donors like FAM (fluorescein). Its broad absorption spectrum (~520–560 nm) and emission properties make it suitable for energy transfer experiments. 2.4 In Vivo Imaging • Applied in cancer research and bacterial transport studies, as demonstrated in field-scale experiments monitoring fluorescently labeled bacterial strains in aquifers. 2.5 Molecular Probes and Beacons • Used to create fluorescent probes for detecting enzyme activity, molecular interactions, and nucleic acid hybridization, owing to its high fluorescence quantum yield and photostability. 3. Advantages • Bright Fluorescence: 5-TAMRA, SE produces intense orange-red fluorescence, readily excited by 532 nm or 546 nm laser lines (e.g., mercury-arc lamps or green He-Ne lasers), making it compatible with standard fluorescence microscopy setups. • pH Insensitivity: Unlike fluorescein, whose fluorescence varies with pH, 5-TAMRA, SE maintains consistent brightness across a wide pH range, enhancing reliability in diverse experimental conditions. • Photostability: Exhibits good resistance to photobleaching compared to fluorescein, allowing for prolonged imaging sessions. • Single-Isomer Purity: The single-isomer form simplifies purification and ensures consistent labeling efficiency, particularly for peptide and protein applications. • Versatility: Compatible with a range of biomolecules, including proteins, peptides, and oligonucleotides, and suitable for multiple techniques like microscopy, flow cytometry, and FRET. 4. Limitations • Lower Quantum Yield: The fluorescence quantum yield of 5-TAMRA, SE conjugates is approximately one-fourth that of fluorescein conjugates, which may result in lower signal intensity in some applications. • Hydrolysis Susceptibility: The succinimidyl ester group is prone to hydrolysis in aqueous solutions, necessitating preparation in anhydrous DMSO and immediate use to maintain labeling efficiency. • Spectral Overlap: While effective in FRET, its broad absorption and emission spectra may lead to spectral overlap with other fluorophores, requiring careful experimental design to avoid crosstalk. • Storage Sensitivity: Requires storage at -20°C, desiccated, and protected from light to prevent degradation. Prolonged exposure to ambient light or moisture can compromise its performance. 5. Practical Considerations 5.1 Storage and Handling • Store desiccated at -20°C, protected from light and moisture, to maintain stability. Stock solutions in anhydrous DMSO can be stored for up to one month under these conditions. • Avoid amine-containing buffers (e.g., Tris, glycine) during conjugation, as they react with the succinimidyl ester, reducing labeling efficiency. 5.2 Conjugation Protocol • Dissolve 5-TAMRA, SE in anhydrous DMSO to prepare a stock solution (e.g., 10 mM). Dilute into an amine-free buffer (pH 7–9) immediately before use. • React with primary amine-containing molecules (e.g., lysine residues on proteins) for 1–2 hours at room temperature, followed by purification (e.g., dialysis or HPLC) to remove unreacted dye. 5.3 Stability Testing • If accidentally exposed to room temperature or light, perform a small-scale positive control experiment to verify functionality, as the dye is stable for days under such conditions. 5.4 Spectral Compatibility • Ensure compatibility with detection systems. 5-TAMRA, SE is spectrally similar to Alexa Fluor® 546, CF™ 543, and MB™ 543, but its excitation/emission maxima (546/579 nm or 553/575 nm) should be matched to the instrument’s filters. 6. Comparison with Alternatives • Fluorescein (FITC): Offers higher quantum yield but is pH-sensitive and less photostable. 5-TAMRA, SE is preferred for applications requiring pH insensitivity and prolonged imaging. • Alexa Fluor® 546: Similar spectral properties but often more expensive. 5-TAMRA, SE is a cost-effective alternative with comparable photostability. • Cy3: Another red-orange fluorophore with slightly different excitation/emission profiles (550/570 nm). Cy3 may offer higher brightness but is more susceptible to photobleaching. • 6-TAMRA, SE: The 6-isomer is preferred for nucleotide labeling, while 5-TAMRA, SE excels in peptide and protein applications due to better HPLC resolution. 5-TAMRA, SE is a robust, versatile fluorophore ideal for labeling peptides, proteins, and occasionally nucleotides, with applications in fluorescence microscopy, FRET, and in vivo imaging. Its bright, pH-insensitive fluorescence, good photostability, and single-isomer purity make it a preferred choice for many biochemical assays, despite its lower quantum yield compared to fluorescein and susceptibility to hydrolysis. Researchers should handle it with care, using anhydrous DMSO and amine-free buffers to maximize labeling efficiency. Compared to alternatives like Alexa Fluor® 546 or Cy3, 5-TAMRA, SE offers a cost-effective, reliable option for fluorescence-based studies. References: 1. Overview of Fluorescent Dyes 2. Evans NA, et al. (2001). Visualizing differences in ligand-induced beta-arrestin-GFP interactions and trafficking between three recently characterized G protein-coupled receptors. J Neurochem 77, 476-85. 3. Nasarabadi S, et al. (1999). Simultaneous detection of TaqMan probes containing FAM and TAMRA reporter fluorophores. Biotechniques 27, 1116-8. |
|
5-TAMRA, SE
For Research & Development use only. Not for testing and/or use on humans.