| Cross Linker (DVB): | 1% |
| Particle Size (mesh): | 100-200 |
| Loading (mmol/g): | 0.4-1.0 |
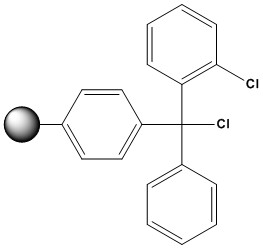
| 2-Chlorotrityl Chloride Resin (2-CTC resin) is a polystyrene-based, acid-labile solid support widely employed in solid-phase peptide synthesis (SPPS) and organic synthesis. Introduced by Barlos et al. in the late 1980s, this resin has become a cornerstone in the preparation of peptides and small organic molecules due to its unique chemical properties and versatility. 1. Structure and Chemical Properties: 2-Chlorotrityl chloride resin consists of a polystyrene or a cross-linked polystyrene matrix (typically 1% or 2% cross-linked with divinylbenzene) functionalized with the 2-chlorotrityl chloride moiety. The key structural features are: • Polystyrene Backbone: Provides the insoluble support necessary for SPS, allowing for easy filtration and washing steps. The degree of cross-linking influences the swelling properties and accessibility of the reactive sites within the resin beads. • Trityl Group: The triphenylmethyl (trityl) group is a sterically hindered and acid-labile protecting group. The steric bulk of the trityl group minimizes side reactions, such as diketopiperazine (DKP) formation, particularly in peptides with C-terminal proline. • Chloride Leaving Group: The chloride atom is the point of attachment for the first building block of the synthesis. It reacts readily with nucleophiles, such as the carboxyl group of an N-protected amino acid or the phosphoramidite of a nucleotide, in the presence of a weak base. • 2-Chloro Substitution: The presence of the chlorine atom at the 2-position of one of the phenyl rings significantly enhances the lability of the trityl-resin bond towards acidic cleavage conditions compared to unsubstituted trityl resins. This increased lability is a crucial advantage of 2-CTC resin. 2. Advantages of 2-Chlorotrityl Chloride Resin: • Mild Cleavage Conditions: The primary advantage of 2-CTC resin is the ability to cleave the synthesized compound from the resin under very mild acidic conditions (typically 1-5% trifluoroacetic acid (TFA) in dichloromethane (DCM) or other suitable solvents). This is crucial for molecules containing acid-sensitive functionalities that might be degraded or modified under the harsher cleavage conditions required for other common resins like Wang resin or Rink amide resin. • High Loading Capacity: 2-CTC resin generally exhibits good loading capacity, meaning a significant amount of the first building block can be attached to the resin. This contributes to higher overall yields of the target molecule. • Versatility: It is compatible with a wide range of synthetic methodologies and can be used for the synthesis of peptides, oligonucleotides, peptoids, and various small organic molecules. • Formation of Acid-Labile Esters or Ethers: The initial attachment of the first building block typically forms an acid-labile ester (when attaching a carboxylic acid) or ether (in some other applications) linkage to the trityl group. This ester or ether bond is the site of cleavage. • Neutralization Step Not Always Necessary: Unlike some other resins where the initial attachment requires deprotonation of the first building block with a strong base, the reaction with 2-CTC resin often proceeds smoothly with a weaker base (like diisopropylethylamine – DIEA) without the need for a separate neutralization step on the resin itself. • Visual Monitoring of Loading: The loading process can sometimes be monitored visually. The reaction of the first building block with the colorless 2-CTC resin often results in a color change (depending on the nature of the building block and reaction conditions), providing a qualitative indication of successful attachment. • Commercially Available: 2-CTC resin is readily available from various chemical suppliers in different particle sizes and loading capacities. 3. Disadvantages and Considerations: • Premature Cleavage: The high lability of the trityl-resin bond can also be a disadvantage. Exposure to even trace amounts of acid during synthesis (e.g., from solvents, reagents, or even prolonged exposure to atmospheric moisture in some cases) can lead to premature cleavage and loss of the growing chain. Therefore, strict anhydrous and acid-free conditions are essential when using 2-CTC resin. • Steric Hindrance: The bulky trityl group can sometimes lead to steric hindrance, potentially affecting the efficiency of subsequent coupling reactions, especially with sterically demanding building blocks. • Swelling Properties: The swelling properties of the polystyrene matrix are crucial for the accessibility of the reactive sites. The choice of solvent can significantly impact swelling, and compatibility with the chosen reaction solvents needs to be considered. • Cost: 2-CTC resin can be more expensive compared to some other commonly used solid supports. • Limited Stability in Certain Solvents: Prolonged exposure to protic solvents, even if nominally anhydrous, should be avoided to minimize the risk of premature cleavage. • Potential for Trityl Cation Formation: Under acidic conditions, the cleavage mechanism involves the formation of a stable trityl carbocation. This carbocation can occasionally react with nucleophiles present in the reaction mixture, leading to side products. 4. Applications in Solid-Phase Synthesis: 2-Chlorotrityl chloride resin finds extensive use in various areas of solid-phase organic synthesis, including: • Solid-Phase Peptide Synthesis (SPPS): 2-CTC resin is renowned for its ability to synthesize protected peptide fragments using the Fmoc/tBu strategy. Its mild cleavage conditions (e.g., 1–5% trifluoroacetic acid (TFA) in DCM with 5% triisopropylsilane (TIS)) preserve acid-sensitive protecting groups like Boc and tBu, making it ideal for convergent peptide synthesis. The resin’s steric hindrance prevents racemization and diketopiperazine (DKP) formation, making it a preferred choice for peptides with C-terminal cysteine or proline. While initially designed for protected fragments, 2-CTC resin can also be used to synthesize fully unprotected peptides by adjusting cleavage conditions (e.g., 50% TFA in DCM). • Oligonucleotide Synthesis: While controlled pore glass (CPG) is the dominant support for oligonucleotide synthesis, 2-CTC resin can be used for specific applications, particularly when mild cleavage is desired. • Small Organic Molecule Synthesis: 2-CTC resin is increasingly employed in the solid-phase synthesis of diverse small organic molecules, providing a convenient handle for building complex structures and facilitating purification. • Linker Chemistry: The 2-chlorotrityl group can be further functionalized with various linkers to introduce specific cleavage sites or functionalities to the synthesized molecule. • Solid-Phase Combinatorial Chemistry: The ease of cleavage under mild conditions makes CTC resin suitable for generating libraries of compounds for drug discovery. 5. Handling and Best Practices: • Storage: Store 2-CTC resin in a cool, dry, and inert atmosphere to prevent degradation and moisture absorption. • Pre-swelling: Before use, the resin should be pre-swollen in a suitable solvent that is compatible with the subsequent coupling reaction. Common pre-swelling solvents include DCM, DMF, and NMP. The swelling time and solvent depend on the resin type and cross-linking. • Loading the First Building Block: The first building block (e.g., an N-protected amino acid) is typically coupled to the resin by reacting its carboxyl group with the chloride leaving group in the presence of a weak base like DIEA or pyridine. The reaction conditions (solvent, temperature, time) need to be optimized for efficient loading. Quantification of the loading capacity after the first coupling step is crucial for accurate yield calculations. • Maintaining Anhydrous and Acid-Free Conditions: Throughout the synthesis, it is paramount to use anhydrous solvents and reagents and to avoid any exposure to acidic conditions that could lead to premature cleavage. • Washing Steps: Thorough washing steps between each synthetic transformation are essential to remove excess reagents and byproducts. The choice of washing solvents should ensure good swelling of the resin and efficient removal of impurities. • Monitoring Reactions: While on-resin reactions can be challenging to monitor directly, techniques like the Kaiser test (for peptide synthesis) or other qualitative tests can provide an indication of the progress of coupling reactions. • Cleavage: Cleavage is typically performed using a dilute solution of TFA (1-5%) in DCM or another compatible solvent. The cleavage time and TFA concentration may need to be optimized depending on the specific molecule and the loading capacity. It is important to collect the cleavage solution carefully and quench the reaction appropriately. • Work-up and Purification: After cleavage, the target molecule needs to be separated from the resin and any remaining reagents or byproducts. Standard work-up procedures, such as evaporation of the solvent and purification by chromatography, are typically employed. 6. Comparison with Other Resins • vs. Wang Resin: Wang resin, another acid-labile support, requires higher TFA concentrations (50–95%) for cleavage, making it less suitable for protected fragments. 2-2-CTC resin’s milder conditions offer a distinct advantage. • vs. Trityl Resin: Standard trityl resin is more acid-labile than 2-CTC, leading to premature cleavage during synthesis. The chlorine substituent in 2-CTC balances stability and lability. • vs. Rink Resin: Rink resin is designed for amide-terminated peptides, whereas 2-CTC targets acid-terminated peptides, serving complementary roles in SPPS. 2-Chlorotrityl chloride resin is a powerful and widely used solid support in organic synthesis, particularly valued for its ability to facilitate the cleavage of synthesized compounds under mild acidic conditions. This feature makes it indispensable for the preparation of acid-sensitive molecules. While the sensitivity to acid requires careful handling and strict reaction conditions, the advantages offered by 2-CTC resin, including its versatility, good loading capacity, and mild cleavage, make it an essential tool for synthetic chemists across various disciplines. Understanding its properties, advantages, disadvantages, and proper handling techniques is crucial for successful implementation in solid-phase synthesis strategies. As the field of SPS continues to evolve, 2-chlorotrityl chloride resin will undoubtedly remain a significant and valuable solid support. References 1. K. Barlos, et al., Tetrahedron Lett., 1989, 30, 3943 2. K. Barlos, et al., Tetrahedron Lett., 1989, 30, 3947 3. K. Barlos, et al., Angew. Chem. Int. Ed. Engl., 1991, 30, 590 4. K. Barlos, et al., Int. J. Pept. Protein Res., 1991, 37, 513 5. K. Barlos, et al., Int. J. Pept. Protein Res., 1991, 38, 562 6. J. Bódi, et. al., Tetrahedron Lett. 1997, 38, 3293 7. J. B. Laursen, et al., Bioorg. Med. Chem. Lett. 2002, 12, 171 8. B. Drouillat, et al., Bioorg. Med. Chem. Lett. 1997, 7, 2247 9. H. Wenschuh, et al., J. Org. Chem., 1995, 60, 405 10. M. A. F. Biabani, et al., Tetrahedron Lett. 2001, 42, 7119 11. Z. Zhu & B. Mckittrick, Tetrahedron Lett., 1998, 39, 7479 12. U. Heinelt, et al., Bioorg. Med. Chem. Lett., 2001, 11, 227 13. M. Cardno, et al., J. Chem. Soc. Chem. Commun., 1995, 2163 14. I. A. Nash, et al. Tetrahedron Lett., 1996, 37, 2625 15. W. J. Hoekstra, et al., Tetrahedron Lett., 1997, 38, 2629 16. J. Perumattan, et al., Mol.Div., 1998, 3, 121 17. J. M. Henlin, et al., J. Pept. Res., 2001, 57, 419 18. S. L. Mellor, et al., Tetrahedron Lett., 1997, 38, 3311. 19. M. M. Meloni & M. Taddei, Org.Lett., 2001, 3, 337. 20. S. Mourtas, et al., Tetrahedron Lett., 2003, 44, 179 21. K. Barlos, et al., Ann. Chem., 1993, 215 22. Peptide Synthesis 23. Overview of Solid Phase Synthesis 24. Fmoc Solid Phase Peptide Synthesis 25. Boc Solid Phase Peptide Synthesis |
|
2-Chlorotrityl Chloride Resin
(100-200 mesh, 0.4-1.0 mmol/g)
For Research & Development use only. Not for testing and/or use on humans.