template
Contents
1. The Fmoc Group
1.1. General Remarks
1.2. Cleavage Procedures
2. Coupling Reaction
2.1. General Remarks
2.2. Activation Methods
2.2.1. Carbodiimide-Carbodiimide/HOBt
2.2.2. Activation by Phosphonium and Uronium/Aminium Salts
2.2.3. Fmoc Amino Acid Active Esters
2.2.4. Fmoc Amino Acid Chlorides and Fluorides
2.3. Monitoring of Coupling and Deblocking
2.3.1. The Kaiser Test
2.3.2. The TNBS Test
2.3.3. The Acetaldehyde/Chloranil Test
2.3.4. The Bromophenol Blue Test
2.3.5. Cleavage of Samples
2.4. Capping
2.5. Aggregation/Difficult Sequences
3. Cleavage from the Resin
3.1. Simultaneous Cleavage from the Resin/Side-Chain Deprotection
3.2. Mix Your Own Cocktail
3.3. Cleavage of Protected Peptide Fragments
4. Side Reactions in Fmoc SPPS
4.1. Diketopiperazine Formation
4.2. Aspartimide Formation
4.3. Transfer of Pmc from Arg to Trp During TFA Deprotection
4.4. 3-(1-Piperidinyl)alanine Formation
4.5. Incomplete Fmoc Deprotection
4.6. Guanylation
5. References
1. The Fmoc Group
1.1. General Remarks
• Due to the development of strategies based on orthogonal protection, Fmoc has become the most important base-labile Nα-protecting group.
• Fmoc is acid-stable, withstands cleavage of Boc/tBu (TFA) and Z/Bzl (HF). Fmoc is stable under the cleavage conditions of • Aloc/All (Pd°). Towards tertiary amines such as DIPEA, pyridine [1]; the relative stability depends on base concentration, • solvent and temperature. Stability towards hydrogenolysis is • controversial [2] and should be evaluated for each individual case. Towards bases, especially secondary amines [1]
• (piperidine>diethylamine); fortunately the Fmoc group is less labile towards primary amines, including the amino group of the amino acid involved in the coupling reaction; premature Fmoc cleavage may nevertheless occur during very slow couplings. N-silylation of the coupling site prevents this side reaction and can enhance the coupling [3].
• The Fmoc group is removed via base-induced β-elimination. As a result dibenzofulvene and carbon dioxide are split off. Secondary bases such as piperidine add to the former molecule whereas bases such as DBU do not react with the dibenzofulvene which has to be removed rapidly from the peptide resin or scavenged by a secondary amine such as piperidine to avoid irreversible attachment to the liberated amino group. Since both cleavage products are strong chromophors the deblocking can be monitored by UV spectroscopy.
By-products generated by the repetitive treatment with base have been described. Aspartimide formation is the best documented side-reaction. Epimerization and subsequent piperidide formation have been detected, even though the bulky tert. butyl group impedes reactions involving the β-carboxy group. Aggregation during chain elongation interferes with the couplings as well as with the Fmoc cleavages. If an incomplete deblocking occurs or is suspected, more active cleavage reagents should be tested.
RECOMMENDED STANDARD PROCEDURE
Fmoc Cleavage
Prewash with DMF (2x)
Treat with piperidine/DMF (1:4), 5 and 10 minutes, 10 ml of reagent/g peptide-resin.
Wash alternately with DMF and IPA until neutral pH.
As mentioned above, the generation and disappearance of Fmoc based chromophors allows the monitoring of the synthesis. Furthermore, samples may be taken to determine the load of Fmoc peptide. The completion of the deprotection reaction may be checked by cleaving samples and analyzing the obtained peptide.
2. Coupling Reaction

RECOMMENDED STANDARD PROCEDURE
TFA Cleavage of In Process Samples
We recommend the use of a standard cleavage cocktail
e.g. TFA/H2O/EDT 90:5:5
Approx. 50 mg of peptide-resin is cleaved for 2 hours with 500 ul of cocktail.
The reaction mixture is filtered into cold MTBE.
The precipitated peptide is isolated by filtration or centrifugation.
The solid is washed with MTBE.
After drying, the crude product is analyzed by HPLC.
3.2. Mix Your Own Cocktail
Sample and bulk cleavages should be performed in a well ventilated hood and protective equipment (gloves, goggles, mask if necessary) worn by the personnel performing the operation.
Starting Cocktail: TFA/water 95:5
Add:
|
Amino acids present |
Recommended scavengers |
|
Cys |
DTE, EDT |
|
Met |
DTE, EDT, 2-mercaptoethanol, ethyl methyl sulfide |
|
Ser, Thr, Tyr |
m- or p-cresol, DTE, EDT |
|
Trp (unprotected) |
DTE, EDT, 2-Me-indole, Ac-Trp-OMe, tryptamine, TIS |

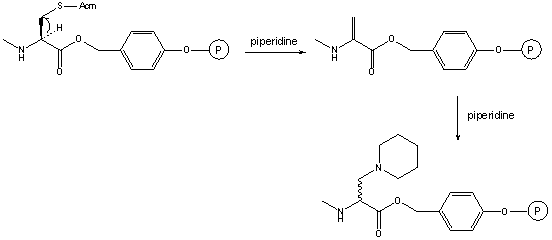
4.4. 3-(1-Piperidinyl)alanine Formation
This modified amino acid is obtained when synthesizing peptides with C-terminal Cys.
The base catalyzed elimination of the suffhydryl protecting group affords dehydroalanine and the subsequent addition of piperidine yields the C-terminally modified peptide [69]. This side reaction is minimized (but not avoided!) when trityl is used for the protection of the C-terminal Cys.
5. References
[1] E. Atherton, RC. Sheppard in The Peptides vol. 9, S. Udenfriend, J. Meienhofer Eds, Academic Press, New York 1987, 1.
[2] E. Atherton, C. Bury, R.C. Sheppard, Bj. Williams, Tetrahedron Lett. (1979) 3041.
[3] H. Wenschuh, M. Beyermann, R Winter, M. Bienert, D. lonescu, L.A. Carpino, Tetrahedron Lett. 37 (1996) 5483.
[4] G.B. Fields in Peptide Synthesis Protocols, M.W. Pennington, B.M. Dunn Eds, Humana Press, Totowa NJ 1994, 17.
[5] J.D. Wade,J. Bedford, R.C. Sheppard, G.W. Tregear, Pept. Res. 4 (1991) 194.
[6] M. Meldal, T. Bielfeldt, S. Peters, Kj. Jensen, H. Paulsen, K. Bock, mt. J. Pept. Protein Res. 43 (1994) 529.
[7] B. Liebe, H. Kunz,Angew. Chem. Tnt. Ed. Engl. 36 (1997) 618.
[8] B.D. Larsen, A. Holm, Int.J. Pept. Protein Res. 43 (1994) 1.
[9] R. Dolling, M. Beyermann,J. Haenel, F. Kernchen, E. Krause, P. Franke, M. Brudel, M. Bienert,J. Chem. Soc., Chem. Comm. (1994) 853.
[10] A. Kapurniotu, C. Ungermann, W. Voelter, in 2nd International Symposium on Innovation and Perspectives in SPPS, Canterbury 1991, R. Epton Ed., Intercept Ltd, Andover 1992, 319.
[11] X. Li, T. Kawakami, S. Aimoto, Tetrahedron Lett. 39 (1998) 8669.
[12] N.L. Benoiton, K. Kuroda, Int.J. Pept. Protein Res. 17(1981)197.
[13] Y. Han, F. Albericio, G. Barany,J. Org. Chem. 62 (1997) 4307.
[14] A. di Fenza, M. Tancredi, C. Galoppini, P. Rovero, Tetrahedron Lett. 39 (1998) 8529.
[15] F. Albericio,J.M. Bofill, A. El-Faham, S.A. Kates,J. Org. Chem. 63 (1998) 9678.
[16] L.A. Carpino,A. El-Faham,J. Org. Chem. 59 (1994) 695.
[17] H. Wenschuh, M. Beyermann, E. Krause, M. Brudel, R. Winter, M. Schumann, L.A. Carpino, M. Bienert,J. Org. Chem. 59 (1994) 3275.
[18] A. Brunissen, M. Ayoub, S. Lavielle, Tetrahedron Lett. 37 (1996) 6713.
[19] RB. MerrifIeld, Biochemistry 3 (1964) 1385.
[20] D. Sarantakis,J. Teichman, El. Lien, R Fenichel, Biochem. Biophys. Res. Comm. 73 (1976) 336.
[21] W. Konig, R. Geiger, Chem. Ber. 103 (1970) 788.
[22] L.A. Carpino,J. Am. Chem. Soc. 115 (1993) 4397.
[23] LA. Carpino, A. El-Faham, C.A. Minor, F. Albericio,J. Chem. Soc., Chem. Comm. (1994) 201.
[24] B. Castro,J.-R Dormoy, G. Evin, C. Selve, Tetrahedron Lett. (1975) 1219.
[25] J. Coste, D. Le-Nguyen, B. Castro, Tetrahedron Lett. 31 (1990) 205.
[26] R Knorr, A. Trzeciak, W. Bannwarth, D. Gillessen, Tetrahedron Lett. 30 (1989) 1927.
[27] L.A. Carpino, D. lonescu, A. El-Faham,J. Org. Chem. 61 (1996) 2460.
[28] L. Kisfaludy, I. Schön, Synthesis (1983) 325.
[29] E. Atherton,J.L. Holder, M. Meldal, R.C. Sheppard, RM. Valerio,J. Chem. Soc., Perkin Trans. 1 (1988) 2887.
[30] E. Atherton, L.R Cameron, R C. Sheppard, Tetrahedron 44 (1988) 843.
[31] L.A. Carpino, A. El-Faham,J. Am. Chem. Soc. 117(1995) 5401.
[32] A. El-Faham, Chemistry Letters (1998) 671.
[33] L.A. Carpino, M. Beyermann, H. Wenschuh, M. Bienert,Acc. Chem. Res. 29 (1996) 268.
[34] L.A. Carpino, Bj. Cohen, K.E. StephensJr, S.Y. Sadat-Aalaee,J.H. Tien, D.C. Langridge, J. Org. Chem. 51 (1986) 3732.
[35] M. Beyermann, M. Bienert, H. Niedrich, L.A. Carpino, D. Sadat-Aalaee,J. Org. Chem. 55 (1990) 721.
[36] E. FaIb, T. Yechezkel, Y. Salitra, C. Gilon,J. Peptide Res. 53 (1999) 507.
[37] E. Kaiser, RL. Colescott, C.D. Bossiriger, P.1. Cook, Anal. Biochem. 34 (1970) 595.
[38] V.K. Sarin, S.B.H. Kent,J.P. Tarn, RB. Merrifleld, Anal. Biochem. 117(1981) 147.
[39] W.S. Hancock,J.E. Battersby, Anal. Biochem. 71 (1976) 260.
[40] T. Vojkovsky, Pept. Res. 8 (1995) 236.
[41] V. Krchnãk,J. Vágner, P. àfár, M. Lebi, Coil. Czech. Chern. Comm. 53 (1988) 2542.
[42] V. Krchnák,J. Vágner, M. LebI, Int.J. Pept. Protein Res. 32 (1988) 415.
[43] B.J. Egner, Gj. Langley, M. Bradley,J. Org. Chem. 60(1995)2652.
[44] G. Talbo,J.D. Wade, N. Dawson, M. Manoussios, G.W. Tregear, Lett. Pept. Sci. 4(1997)121.
[45] C.L. Brummel,J.C. Vickerman, S.A. Carr, M.E. Hemling, G.D. Roberts, W.Johnson, J. Weinstock, D. Gaitanopoulos, Sj. Benkovic, N. Winograd, Anal. Chern. 68 (1996) 237.
[46] E. Giralt,J. Rizo, E. Pedroso, Tetrahedron 40 (1984) 4141.
[47] S.M. Meister, S.B.H. Kent, in Peptides, Chemistry Structure and Biology, Proceedings 8th American Peptide Symposium Tucson, 1983, Vj. Hruby, D.H. Rich Eds, Pierce Chemical Company, Rockford Il 1983, 103.
[48] E. Oliveira, A. Miranda, F. Albericio, D. Andreu, A.C.M. Paiva, C.R. Nakaie, M. Torninaga, J. Pept. Res. 49 (1997) 300.
[49] M.W. Pennington, I. Zaydenberg, M.E. Byrnes, RS. Norton, W.R Kern, Tnt. J. Pept. Protein Res. 43 (1994) 463.
[50] L. Zhang, C. Goldammer, B. Henkel, F. Zühl, G. Panhaus, G.Jung, E. Bayer, in 3rdlnternational Symposium on Innovation and Perspectives in SPPS, Oxford UK, R Epton Ed., Mayflower Scientific Ltd, Birmingham 1994, 711.
[51] L.M. Varanda, M.T.M. Miranda,J. Pept. Res. 50 (1997) 102.
[52] H.G. Chao, M.S. Bernatowicz, G.R. Matsueda,J. Org. Chem. 58 (1993) 2640.
[53] M. Quibell, Tjohnson, in Fmoc Solid Phase Peptide Synthesis-A Practical Approach, W.C. Chan, P.D. White Eds, Oxford University Press 2000, 115, and references therein.
[54] C. Hyde, Tj.Johnson, D. Owen, M. Quibell, R.C. Sheppard, Int.J. Pept. Protein Res. 43 (1994) 431.
[55] M. Mutter, A. Nefzi, T. Sato, X. Sun, F. Wahl,T. Wohr, Pept. Res. 8 (1995) 145.
[56] D.S. King, C.G. Fields, G.B. Fields, Tnt.J. Pept. Protein Res. 36 (1989) 255.
[57] F. Dick in Peptide Synthesis Protocols, M. Pennington and B.M. Dunn Eds, Humana Press, Totowa NJ 1994, 63.
[58] E. Atherton, R.C. Sheppard, P. Ward,J. Chem. Soc., Perkin Trans. I (1985) 2065.
[59] D. Pearson, M. Blanchette, M.L. Baker, C.A. Guidon, Tetrahedron Lett. 30 (1989) 2739.
[60] C.G. Fields, G.B. Fields, Tetrahedron Lett. 34 (1993) 6661.
[61] N.A. Sole, G. Barany,J. Org. Chem. 57(1992) 5399.
[62] K. Barbs, D. Gatos, J.Kallitsis, G. Papaphotiu, P. Sotiriu, Y. Wenging, W. Schafer, Tetrahedron Lett. 30(1989) 3943.
[63] R Bolihagen, M. Schmiedberger, K. Barbs, E. Grell,J. Chem. Soc., Chem. Comm. (1994) 2559.
[64] R Dolling, M. Beyermann,J. Haenel, F. Kernchen, E. Krause, P. Franke, M. Brudel,
M. Bienert in 3rd International Symposium on Innovation and Perspectives in SPPS, Oxford UK, R. Epton Ed., Mayflower Scientific Ltd, Birmingham 1994,489.
[65] Y. Yang, W.V. Sweeney, K. Schneider, S. Thornqvist, B.T. Chait, J.P. Tam, Tetrahedron Lett. 52 (1994) 9689.
[66] J.L. Lauer, C.G. Fields, G.B. Fields, Lett. Pept. Sci. 1 (1994) 197.
[67] M. Quibell, D. Owen, L.C. Packman, Tj.Johnson,J. Chem. Soc., Chem. Comm. (1994) 2343.
[68] A. Stierandova, N.F. Sepetov, G.V. Nikiforovich, M. LebI, Int.J. Pept. Protein Res. 43 (1994) 31.
[69] J. Lukszo, D. Patterson, F. Albericio, S.A. Kates, Lett. Pept. Sci. 3 (1996) 157.


