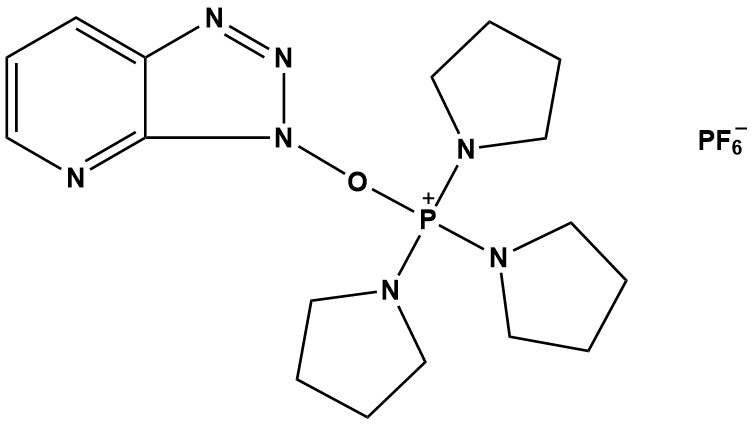
| Synonym: | (3-Hydroxy-3H-1,2,3-triazolo[4,5-b]pyridinato-O)tri-1-pyrrolidinylphosphonium hexafluorophosphate |
| CAS #: | 156311-83-0 |
| Molecular Formula: | C17H27F6N7OP2 |
| Molecular Weight: | 521.4 |
| PyAOP (CAS #: 156311-83-0) is a highly efficient phosphonium-based coupling reagent widely employed in peptide synthesis and related amide bond formation reactions. As a member of the phosphonium family of coupling reagents, PyAOP offers exceptional reactivity, minimal racemization, and compatibility with a broad range of substrates. 1. Introduction Peptide synthesis represents one of the most fundamental and challenging tasks in organic chemistry, biochemistry, and pharmaceutical sciences. The efficient formation of amide bonds between amino acids requires coupling reagents that can activate carboxylic acids while minimizing side reactions such as racemization, which can compromise the stereochemical integrity of the final product. Over the past several decades, numerous coupling reagents have been developed, each offering distinct advantages and limitations. PyAOP emerged in the early 1990s as part of a new generation of phosphonium-based coupling reagents designed to address the shortcomings of earlier reagents. Its development was driven by the need for more efficient activation, reduced racemization, and improved compatibility with sensitive functional groups. Today, PyAOP stands as one of the premier choices for peptide chemists and synthetic organic chemists working on complex amide bond formations. 2. Chemical Structure and Physical Properties 2.1 Molecular Structure PyAOP possesses a complex molecular structure comprising several key components that contribute to its reactivity and selectivity. The reagent consists of: • A phosphonium center with three pyrrolidino substituents • A 7-azabenzotriazol-1-yloxy (OAt) leaving group • A hexafluorophosphate (PF6⁻) counterion The molecular formula is C17H27F6N7OP2 with a molecular weight of 521.4 g/mol. The phosphonium core provides electrophilic activation of the carboxylic acid, while the OAt moiety serves as an excellent leaving group that facilitates nucleophilic substitution. The three pyrrolidino groups enhance solubility in organic solvents and contribute to the reagent’s stability. 2.2 Physical Properties Appearance: White to off-white crystalline powder Melting Point: Decomposes above 160°C Solubility: Highly soluble in DMF, DCM, acetonitrile; sparingly soluble in diethyl ether Storage Conditions: Store at -20°C under inert atmosphere; moisture sensitive Stability: Stable when stored properly; decomposes in the presence of moisture 3. Mechanism of Action 3.1 General Activation Mechanism PyAOP functions as an in situ activation reagent for carboxylic acids, converting them into highly reactive acyloxyphosphonium intermediates. The mechanism proceeds through the following steps: Step 1: Deprotonation – A tertiary amine base (commonly DIPEA or NMM) deprotonates the carboxylic acid to form a carboxylate anion. Step 2: Nucleophilic attack – The carboxylate anion attacks the electrophilic phosphorus center of PyAOP, displacing the OAt leaving group. Step 3: Formation of activated ester – The resulting acyloxyphosphonium intermediate can either react directly with the amine nucleophile or undergo rearrangement to form a HOAt active ester. Step 4: Aminolysis – The amine nucleophile attacks the activated carbonyl, displacing the activating group and forming the desired amide bond. 3.2 Suppression of Racemization One of the most critical aspects of peptide synthesis is the preservation of stereochemical integrity at the α-carbon of amino acids. Racemization occurs through the formation of an oxazolone intermediate, which possesses an acidic proton at the α-position that can undergo enolization. PyAOP minimizes racemization through several mechanisms: • The OAt moiety is a poor leaving group from oxazolone intermediates, thus discouraging oxazolone formation. • HOAt, which is generated during the coupling reaction, acts as a weak nucleophile and weak base, reducing the propensity for base-catalyzed enolization. • The use of weak, non-nucleophilic bases such as DIPEA further minimizes racemization risks. Studies have demonstrated that PyAOP exhibits racemization levels below 0.5% for most amino acids, even for problematic residues such as cysteine and histidine. 4. Applications in Peptide Synthesis 4.1 Solid-Phase Peptide Synthesis (SPPS) PyAOP is extensively utilized in both Fmoc and Boc solid-phase peptide synthesis strategies. Its compatibility with automated synthesizers and high coupling efficiency make it a preferred choice for: • Routine peptide assembly on polystyrene and PEG-based resins • Coupling of sterically hindered amino acids • Synthesis of peptides containing sensitive residues (Cys, His, Trp) • Preparation of cyclic peptides and constrained structures Typical coupling conditions involve 3-5 equivalents of PyAOP relative to the resin-bound amino acid, 6-10 equivalents of DIPEA, and reaction times of 30-60 minutes in DMF or NMP. 4.2 Solution-Phase Peptide Synthesis While SPPS dominates modern peptide synthesis, solution-phase methods remain valuable for large-scale production and specialized applications. PyAOP is highly effective in solution-phase couplings, offering: • Rapid reaction kinetics, often achieving completion in 15-30 minutes • High yields (typically 85-95%) • Minimal side product formation • Ease of purification due to water-soluble byproducts 4.3 Specialized Applications Beyond standard peptide synthesis, PyAOP finds utility in: Peptide-protein conjugation: PyAOP facilitates the attachment of peptides to protein scaffolds for vaccine development and therapeutic applications. Lipopeptide synthesis: The reagent is compatible with lipidated amino acids, enabling the preparation of lipopeptides with antimicrobial and immunomodulatory properties. Peptide nucleic acid (PNA) synthesis: PyAOP is employed in the synthesis of PNA oligomers, which are peptide-based DNA analogs. Macrocyclization reactions: PyAOP can mediate head-to-tail cyclization of linear peptides under high-dilution conditions. Synthesis of β-peptides and peptidomimetics: The reagent’s low racemization profile makes it suitable for coupling non-proteinogenic amino acids. 5. Advantages of PyAOP 5.1 High Coupling Efficiency PyAOP exhibits exceptional reactivity, enabling rapid and complete coupling reactions. This is particularly advantageous for: • Hindered couplings involving N-methylated amino acids, β-branched residues (Val, Ile, Thr), and proline • Automated peptide synthesis where rapid reaction kinetics are essential • Scale-up processes where reaction time directly impacts production costs 5.2 Minimal Racemization As discussed in Section 3.2, PyAOP demonstrates exceptionally low racemization rates. This is crucial for maintaining the biological activity and structural integrity of synthesized peptides. Comparative studies have shown that PyAOP produces less racemization than many alternative reagents, including DCC, EDC, and certain uronium reagents. 5.3 Broad Functional Group Compatibility PyAOP is compatible with a wide range of functional groups and protecting group strategies, including: • Fmoc/tBu protecting groups (standard in modern SPPS) • Boc/Bzl protecting groups (classical SPPS) • Acid-labile linkers and resins • Sensitive side chains (indole, thioether, imidazole) 5.4 Clean Reaction Profile PyAOP generates water-soluble byproducts (phosphine oxide and HOAt derivatives) that are easily removed during aqueous workup or resin washing. This simplifies purification and reduces the risk of byproduct contamination in the final peptide. 5.5 Stability and Shelf Life When stored properly under inert atmosphere at -20°C, PyAOP remains stable for extended periods (typically >2 years). This contrasts favorably with some uronium reagents that can decompose upon storage or exposure to moisture. 6. Limitations and Considerations 6.1 Cost PyAOP is more expensive than some alternative coupling reagents, particularly carbodiimides such as DCC and EDC. For large-scale syntheses or routine applications where racemization is not a concern, more economical reagents may be preferred. 6.2 Moisture Sensitivity PyAOP is highly sensitive to moisture and must be handled under anhydrous conditions. Exposure to atmospheric moisture can lead to rapid decomposition and reduced coupling efficiency. Rigorous exclusion of water is essential, particularly in humid environments. 6.3 Potential for Guanidination Under certain conditions, particularly in the presence of excess base, PyAOP can cause guanidination of lysine residues, leading to the formation of homoarginine. This side reaction can be minimized by using appropriate base stoichiometry and shorter reaction times. 6.4 Incompatibility with Strong Nucleophiles PyAOP can react with strong nucleophiles such as thiols and primary alcohols, potentially leading to side reactions. When coupling peptides containing free cysteine residues, appropriate protecting groups must be employed. 7. Comparison with Other Coupling Reagents The landscape of peptide coupling reagents is diverse, with numerous options available. The following table compares PyAOP with commonly used alternatives: PyAOP Type: Phosphonium Racemization: Very Low Efficiency: Very High Cost: High Applications: SPPS, hindered couplings HATU Type: Uronium Racemization: Very Low Efficiency: Very High Cost: High Applications: General SPPS HBTU Type: Uronium Racemization: Low Efficiency: High Cost: Moderate Applications: General SPPS DCC Type: Carbodiimide Racemization: Moderate Efficiency: Moderate Cost: Low Applications: Solution phase EDC Type: Carbodiimide Racemization: Moderate Efficiency: Moderate Cost: Low Applications: Bioconjugation PyBOP Type: Phosphonium Racemization: Low Efficiency: High Cost: Moderate Applications: General SPPS PyAOP and HATU are considered the gold standards for minimizing racemization in demanding peptide syntheses. PyAOP is often preferred for couplings involving histidine and cysteine residues due to its superior compatibility with these amino acids. 8. Experimental Protocols 8.1 General Procedure for SPPS Coupling Materials: • Fmoc-protected amino acid (3-5 eq) • PyAOP (3-5 eq) • DIPEA (6-10 eq) • DMF (anhydrous) • Resin-bound peptide Procedure: 1. Swell the resin in DMF for 15 minutes. 2. Remove the N-terminal Fmoc protecting group using 20% piperidine in DMF (2 × 5 min). 3. Wash the resin thoroughly with DMF (5 × 1 min). 4. In a separate vessel, dissolve the Fmoc-amino acid in minimal DMF. 5. Add PyAOP and DIPEA to the amino acid solution and mix briefly (10-30 seconds). 6. Immediately transfer the activated amino acid solution to the resin. 7. Agitate the reaction mixture for 30-60 minutes at room temperature. 8. Drain the coupling solution and wash the resin with DMF (5 × 1 min). 9. Assess coupling completion using the Kaiser test or other suitable method. 10. If coupling is incomplete, repeat the coupling procedure. 8.2 Solution-Phase Coupling Materials: • Protected carboxylic acid (1 eq) • Protected amine (1.1-1.2 eq) • PyAOP (1.1 eq) • DIPEA (2-3 eq) • DCM or DMF (anhydrous) Procedure: 1. Dissolve the carboxylic acid in anhydrous DCM or DMF. 2. Cool the solution to 0°C if working with highly reactive substrates. 3. Add PyAOP followed by DIPEA and stir for 2-5 minutes to activate the carboxylic acid. 4. Add the amine nucleophile and allow the reaction to warm to room temperature. 5. Monitor the reaction by TLC or HPLC (typical reaction time: 15-60 minutes). 6. Quench the reaction with saturated aqueous NaHCO₃ or dilute HCl (depending on the substrate). 7. Extract the product into an appropriate organic solvent. 8. Wash the organic phase, dry over anhydrous Na₂SO₄, and concentrate under reduced pressure. 9. Purify the product by column chromatography or recrystallization. 8.3 Troubleshooting Guide Incomplete coupling Possible Cause: Degraded PyAOP or insufficient activation time Solution: Use fresh reagent; extend coupling time; increase equivalents Racemization Possible Cause: Excessive base or prolonged activation Solution: Reduce base equivalents; minimize pre-activation time Guanidination Possible Cause: Excess PyAOP or extended reaction time Solution: Use stoichiometric PyAOP; shorten reaction time Low yield Possible Cause: Moisture contamination Solution: Ensure anhydrous conditions; use freshly dried solvents 9. Recent Advances and Future Perspectives 9.1 Microwave-Assisted Synthesis Recent studies have explored the use of PyAOP in microwave-assisted peptide synthesis. This approach combines the high reactivity of PyAOP with the accelerated kinetics provided by microwave irradiation, enabling: • Coupling times reduced to 2-5 minutes • Improved yields for difficult sequences • Reduced reagent consumption • Enhanced compatibility with automated synthesizers Preliminary results suggest that microwave conditions do not increase racemization when PyAOP is employed, making this combination particularly attractive for complex peptide syntheses. 9.2 Flow Chemistry Applications Continuous flow synthesis has emerged as a powerful platform for peptide production. PyAOP’s rapid kinetics and clean reaction profile make it well-suited for flow chemistry applications. Advantages of flow-based PyAOP couplings include: • Precise control of reaction parameters (temperature, residence time, stoichiometry) • Reduced reaction volumes and improved safety • Potential for in-line purification and monitoring • Scalability from milligrams to kilograms Several research groups have demonstrated successful flow-based peptide synthesis using PyAOP, achieving comparable or superior results to batch processes. 9.3 Green Chemistry Initiatives The chemical industry is increasingly focused on sustainability and reducing environmental impact. Efforts to improve the green credentials of PyAOP-mediated peptide synthesis include: • Development of recyclable phosphonium reagents • Use of greener solvents (e.g., 2-methyltetrahydrofuran, cyclopentyl methyl ether) • Solvent recovery and recycling strategies • Enzyme-assisted deprotection to reduce chemical waste While PyAOP itself is not recyclable in its current form, ongoing research aims to develop next-generation phosphonium reagents that retain PyAOP’s desirable properties while addressing sustainability concerns. 9.4 Expanding Applications Beyond Peptides Although PyAOP was developed primarily for peptide synthesis, its utility has expanded into other areas of synthetic chemistry: Polymer synthesis: PyAOP has been employed in the synthesis of polyamides and polyesters with well-defined structures. Natural product synthesis: The reagent has been used in key amide bond-forming steps in the total synthesis of complex natural products. Drug discovery: PyAOP facilitates the rapid assembly of compound libraries for screening programs. Materials science: PyAOP enables the functionalization of surfaces and nanoparticles with peptide and protein motifs. 10. Conclusions PyAOP stands as a cornerstone reagent in modern peptide synthesis, offering an exceptional balance of reactivity, selectivity, and minimal racemization. Its phosphonium structure endows it with high coupling efficiency, particularly for challenging substrates, while the OAt leaving group provides a clean reaction profile with minimal side products. The widespread adoption of PyAOP in both academic and industrial settings reflects its reliability and versatility. From routine solid-phase peptide synthesis to complex macrocyclizations and specialized applications in bioconjugation and materials science, PyAOP has proven to be an indispensable tool. While certain limitations exist—including cost, moisture sensitivity, and potential for guanidination under suboptimal conditions—these can be effectively managed through proper handling and optimization of reaction conditions. The reagent’s advantages far outweigh these considerations for applications where stereochemical integrity and high coupling efficiency are paramount. Looking forward, PyAOP is poised to benefit from advances in enabling technologies such as microwave synthesis, flow chemistry, and automation. These developments promise to further enhance the efficiency, scalability, and sustainability of PyAOP-mediated peptide synthesis. Additionally, efforts to develop greener variants and expand applications into non-peptide domains will likely extend the impact of this important reagent. For researchers engaged in peptide chemistry, PyAOP represents a trusted choice backed by decades of successful applications. Its continued evolution alongside emerging synthetic methodologies ensures that PyAOP will remain at the forefront of amide bond formation chemistry for years to come. References 1. Coste, J.; Le-Nguyen, D.; Castro, B. PyBOP: A new peptide coupling reagent devoid of toxic by-product. Tetrahedron Lett. 1990, 31, 205-208. 2. Albericio, F.; Bofill, J. M.; El-Faham, A.; Kates, S. A. Use of onium salt-based coupling reagents in peptide synthesis. J. Chem. Soc., Perkin Trans. 1 1998, 241-250. 3. El-Faham, A.; Albericio, F. Peptide coupling reagents, more than a letter soup. Chem. Rev. 2011, 111, 6557-6602. 4. Valeur, E.; Bradley, M. Amide bond formation: beyond the myth of coupling reagents. Chem. Soc. Rev. 2009, 38, 606-631. 5. Montalbetti, C. A. G. N.; Falque, V. Amide bond formation and peptide coupling. Tetrahedron 2005, 61, 10827-10852. 6. Han, S.-Y.; Kim, Y.-A. Recent development of peptide coupling reagents in organic synthesis. Tetrahedron 2004, 60, 2447-2467. 7. Dunetz, J. R.; Magano, J.; Weisenburger, G. A. Large-scale applications of amide coupling reagents for the synthesis of pharmaceuticals. Org. Process Res. Dev. 2016, 20, 140-177. 8. Subirós-Funosas, R.; Prohens, R.; Barbas, R.; El-Faham, A.; Albericio, F. Oxyma: An efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion. Chem. Eur. J. 2009, 15, 9394-9403. 9. Carpino, L. A. 1-Hydroxy-7-azabenzotriazole. An efficient peptide coupling additive. J. Am. Chem. Soc. 1993, 115, 4397-4398. 10. Marder, O.; Albericio, F. Industrial application of coupling reagents in peptides. Chim. Oggi 2003, 21, 35-40. 11. Jaradat, D. M. M. Thirteen decades of peptide synthesis: key developments in solid phase peptide synthesis and amide bond formation utilized in peptide ligation. Amino Acids 2018, 50, 39-68. 12. Paradís-Bas, M.; Tulla-Puche, J.; Albericio, F. The road to the synthesis of “difficult peptides”. Chem. Soc. Rev. 2016, 45, 631-654. 13. de la Torre, B. G.; Albericio, F. Peptide therapeutics 2.0. Molecules 2020, 25, 2293. 14. Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4-27. 15. Isidro-Llobet, A.; Kenworthy, M. N.; Mukherjee, S.; Kopach, M. E.; Wegner, K.; Gallou, F.; Smith, A. G.; Roschangar, F. Sustainability challenges in peptide synthesis and purification: from R&D to production. J. Org. Chem. 2019, 84, 4615-4628. |
|
PyAOP
For Research & Development use only. Not for testing and/or use on humans.