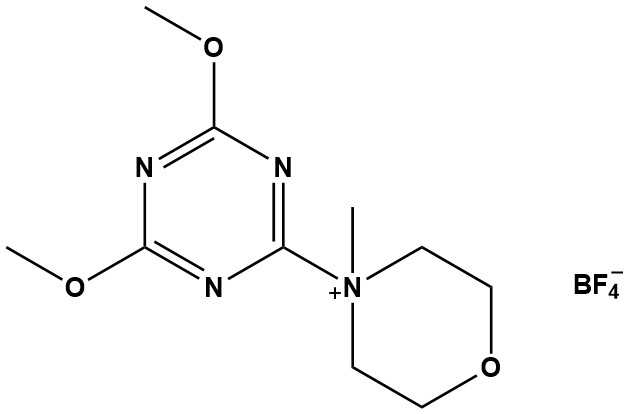
| Synonym: | 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium tetrafluoroborate |
| CAS #: | 293311-03-2 |
| Molecular Formula: | C10H17BF4N4O3 |
| Molecular Weight: | 328.1 |
| MMTM (CAS #: 293311-03-2) represents an advanced member of the triazine-based coupling reagent family. As the tetrafluoroborate salt variant of DMTMM (the chloride salt), MMTM offers enhanced stability and improved performance characteristics in peptide synthesis, amide bond formation, and esterification reactions. This comprehensive review examines the chemical properties, synthesis mechanisms, applications, and safety considerations of this important synthetic reagent. 1. Chemical and Physical Properties 1.1 Molecular Characteristics CAS Number: 293311-03-2 Molecular Formula: C10H17BF4N4O3 Molecular Weight: 328.1 g/mol Appearance: White crystalline powder Storage Conditions: 2-8°C, protected from moisture 1.2 Structural Features MMTM features a quaternary morpholinium cation bonded to a dimethoxy-substituted triazine ring, paired with a tetrafluoroborate counterion. The tetrafluoroborate anion (BF4⁻) is non-nucleophilic, which provides enhanced stability compared to the chloride salt (DMTMM). This structural modification improves reagent performance in various reaction media and extends shelf life under proper storage conditions. 2. Synthesis and Preparation 2.1 Synthetic Route MMTM is prepared through a two-step synthesis. First, 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT) is reacted with N-methylmorpholine (NMM) to form the intermediate 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM). This chloride salt is then subjected to anion exchange with lithium or silver tetrafluoroborate to yield the tetrafluoroborate salt, MMTM. The process was first reported in 2005 by Kamiński and colleagues, who designed this new generation of coupling reagents based on the concept of superactive esters. 2.2 Advantages of the Tetrafluoroborate Salt The tetrafluoroborate counterion offers several advantages over the chloride salt: • Enhanced chemical stability in both solid state and solution • Non-nucleophilic nature prevents unwanted side reactions • Broader solvent compatibility, particularly in organic media • Reduced hygroscopicity compared to halide salts • Extended shelf life under appropriate storage conditions 3. Mechanism of Action 3.1 The Superactive Ester Mechanism MMTM operates through a well-characterized superactive ester mechanism that proceeds via nucleophilic aromatic substitution (SNAr). The carboxylic acid substrate undergoes nucleophilic attack on the triazine ring of MMTM, displacing one of the methoxy groups and forming a highly reactive triazine ester intermediate. This activated ester is then attacked by a nucleophile (amine for amide formation, alcohol for ester formation). The departure of the triazine leaving group is energetically favorable as it rearranges into a stable, chemically inert triazinone structure, driving the reaction forward and minimizing side product formation. 3.2 Key Mechanistic Advantages • No requirement for pre-activation steps • Rapid reaction kinetics enabling high throughput synthesis • Minimal racemization during peptide coupling • Clean byproduct profile facilitating purification • High coupling yields even with sterically hindered substrates 4. Applications in Organic Synthesis 4.1 Peptide Synthesis MMTM has established itself as a premier coupling reagent for both solution-phase and solid-phase peptide synthesis (SPPS). Research has demonstrated that MMTM achieves coupling yields of 80-100% with high enantiomeric purity when synthesizing dipeptides from natural and unnatural amino acids, including sterically hindered substrates. Comparative studies have shown that automated SPPS using MMTM produces purer products than established reagents such as TBTU or PyBOP. Manual SPPS proceeds significantly faster with MMTM than with HATU or TBTU, and the reagent is effective for on-resin head-to-tail cyclization of constrained cyclopeptides. 4.2 Amide Bond Formation Beyond peptide synthesis, MMTM serves as an excellent general coupling reagent for amide formation from carboxylic acids and amines. The reaction is typically a straightforward one-step process that delivers high yields. MMTM has proven particularly advantageous for sterically hindered amines where traditional coupling agents may fail or give poor yields. The water-soluble byproducts are easily removed through simple washing procedures, significantly simplifying purification. 4.3 Bioconjugation Chemistry MMTM and its chloride analog DMTMM have found important applications in bioconjugation chemistry, including peptide-oligonucleotide conjugate synthesis, hyaluronic acid derivatization for hydrogel formation, polysaccharide ligation, and protein modification. In comparative studies for peptide-oligonucleotide conjugation, water-soluble reagents including DMTMM produced markedly higher yields than hydrophobic alternatives like PyBOP and HBTU. Studies have shown DMTMM superior to EDC/NHS for hyaluronic acid ligation, with higher yields and no requirement for strict pH control. 4.4 Materials Science Applications Research has explored MMTM-type reagents in the preparation of organic-inorganic hybrid nanocomposites. Studies on epoxy/amine cure reactions with organically modified montmorillonites have shown that these coupling reagents can influence reaction mechanisms, molecular mobility during curing, and the final glass transition temperatures of nanocomposite materials. 5. Comparison with Other Coupling Reagents Traditional carbodiimide coupling reagents such as DCC, DIC, and EDC have been workhorses of synthetic chemistry for decades. However, MMTM offers simplified purification since carbodiimides produce urea byproducts that can be difficult to remove. Compared to phosphonium and uronium reagents like PyBOP, TBTU, and HATU, direct comparative studies have shown that MMTM achieves comparable or superior yields in peptide synthesis while being more cost-effective for large-scale applications. The primary difference between MMTM and DMTMM lies in the counterion, with MMTM exhibiting enhanced stability, reduced hygroscopicity, and improved compatibility with a wider range of organic solvents. 6. Experimental Considerations 6.1 Reaction Conditions and Solvent Selection MMTM coupling reactions typically proceed under mild conditions at room temperature or slightly elevated temperatures. Stoichiometric amounts of reagents (1.0-2.0 equivalents) are generally employed without time-consuming optimization. The reagent is compatible with Z-, Boc-, and Fmoc-protecting group strategies. While DMTMM (the chloride salt) is notably water-soluble, the tetrafluoroborate salt MMTM shows enhanced solubility in organic solvents including DMF, DCM, THF, and acetonitrile, making it particularly suitable for anhydrous conditions. 6.2 Workup and Purification One of MMTM’s significant practical advantages is the ease of product purification. The triazinone byproduct and other reaction byproducts are typically water-soluble and can be removed by simple aqueous extraction or washing. The clean byproduct profile often results in high crude product purity, sometimes eliminating the need for extensive chromatographic purification. 7. Conclusions MMTM represents a highly effective and versatile coupling reagent for modern organic synthesis. Its superactive ester mechanism provides rapid, high-yielding coupling with minimal racemization, making it particularly valuable for peptide synthesis. Key advantages include efficient activation without pre-activation, excellent performance with sterically hindered substrates, clean byproduct profiles facilitating purification, and cost-effectiveness compared to many alternative coupling reagents. Applications span peptide and protein chemistry, bioconjugation, materials science, and general organic synthesis. While appropriate safety precautions are required due to its moderate skin sensitization potential, MMTM has become a valuable tool in the synthetic chemist’s arsenal. References 1. Kamiński et al. (2005). N-Triazinylammonium Tetrafluoroborates. A New Generation of Efficient Coupling Reagents Useful for Peptide Synthesis. J. Am. Chem. Soc., 127(48), 16912-16920. 2. Kunishima et al. (1999). 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent. Tetrahedron, 55(46), 13159-13170. 3. Falchi et al. (2000). DMTMM: A Valuable Alternative to PyBOP for Solid Phase Peptide Synthesis. Synlett, 275-277. 4. D’Este et al. (2014). A systematic analysis of DMTMM vs EDC/NHS for ligation of amines to hyaluronan in water. Carbohydr. Polym., 108, 239-246. 5. Raw, S. A. (2009). An improved process for the synthesis of DMTMM-based coupling reagents. Tetrahedron Lett., 50(8), 946-948. |
|
MMTM
For Research & Development use only. Not for testing and/or use on humans.