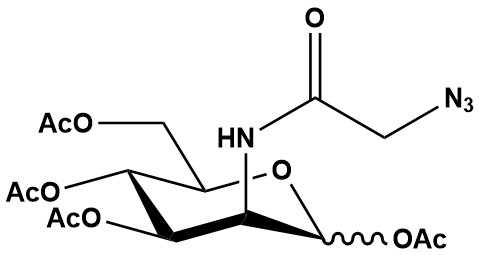
| Synonym: | N-azidoacetylmannosamine-tetraacylated |
| CAS #: | 361154-30-5 |
| Molecular Formula: | C16H22N4O10 |
| Molecular Weight: | 430.4 |
| Ac4ManNAz (N-Azidoacetylmannosamine-tetraacetylated) is a critical metabolic chemical reporter (MCR) used in glycobiology and chemical biology for the study of sialic acids and sialoglycoconjugates. Like Ac4GalNAz, it leverages the principles of metabolic glycoengineering and bioorthogonal click chemistry to enable the visualization, isolation, and manipulation of these important cellular components. 1. Mechanism of Action and Metabolic Labeling The mechanism of Ac4ManNAz mirrors that of other acetylated azidosugars: 1.1 Cell Permeability The acetyl groups on Ac4ManNAz render it lipophilic, allowing it to easily traverse the lipid bilayer of cell membranes. 1.2 Intracellular Deacetylation Once inside the cell, ubiquitous intracellular carboxyesterases cleave the acetyl groups, unmasking the hydroxyl groups. 1.3 Metabolic Incorporation into Sialic Acid Pathway The deacetylated N-azidoacetylmannosamine (ManNAz) then enters the sialic acid biosynthetic pathway. ManNAz acts as an analog of natural N-acetylmannosamine (ManNAc), a precursor to sialic acid. It is converted through a series of enzymatic steps (including phosphorylation by ManNAc kinase and condensation with pyruvate by sialic acid synthase) into N-azidoacetylsialic acid (SiaNAz), which is then converted into CMP-SiaNAz. This azide-modified activated sugar is then incorporated by sialyltransferases into newly synthesized sialoglycoconjugates (glycoproteins and glycolipids), primarily located on the cell surface and in secreted proteins. 1.4 Bioorthogonal Ligation (Click Chemistry) The azide tag on the metabolically incorporated sialoglycoconjugates serves as a bioorthogonal handle for subsequent “click chemistry” reactions. • Copper-catalyzed Azide-Alkyne Cycloaddition (CuAAC): Highly efficient, but requires a copper(I) catalyst, which can be toxic for live cells. • Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC) or Copper-Free Click Chemistry: Utilizes strained cyclooctynes (e.g., DBCO, BCN) that react with azides without the need for a catalyst, making it suitable for live-cell imaging and in vivo applications. • Reporter molecules (e.g., fluorescent dyes, biotin, affinity tags) bearing an alkyne or cyclooctyne can be selectively attached to the azide-labeled sialoglycans. 2. Applications Ac4ManNAz is a cornerstone tool for studying sialylation, a crucial glycosylation event involved in numerous biological processes: • Sialoglycan Visualization and Imaging: Used to fluorescently label cell surface sialoglycoproteins and glycolipids, allowing their detection and visualization by techniques such as fluorescence microscopy, flow cytometry, and in gel fluorescence. This facilitates studies of sialic acid distribution, dynamics, and trafficking. • Glycoproteomics and Glycolipidomics: Enables the selective enrichment and identification of sialylated proteins and lipids from complex biological samples. Following labeling and click chemistry with a biotinylated alkyne, tagged biomolecules can be purified using streptavidin and identified by mass spectrometry. • Studying Sialyltransferase Activity: Can be employed to investigate the substrate specificities and activities of various sialyltransferases, the enzymes responsible for adding sialic acids to glycans. • Investigating Sialylation in Disease: Sialylation patterns often change in various diseases, including cancer, inflammation, and neurodegenerative disorders. Ac4ManNAz provides a means to study these alterations, identify potential biomarkers, and understand disease mechanisms. • Cell Surface Engineering: Allows for the introduction of azide handles onto cell surfaces, which can then be used to attach a wide range of molecules (e.g., targeting ligands, therapeutic agents, imaging probes) for cell-based therapies, diagnostics, and biomaterial development. • Cell Tracking and Homing: Azide-labeled cells can be tracked in vivo by reacting them with imaging probes, offering insights into cell migration, engraftment, and biodistribution in animal models. • Drug Delivery and Diagnostics: Contributes to the design of targeted drug delivery systems and diagnostic assays by leveraging the specific labeling of sialylated structures, which can serve as disease-specific targets. • GlycoRNA Discovery: Recent groundbreaking research has revealed that Ac4ManNAz can also be incorporated into a newly discovered class of glyco-modified RNA (glycoRNA), primarily within exosomes. This opens up entirely new avenues for understanding RNA modifications and their biological roles. 3. Advantages • High Specificity for Sialic Acids: Ac4ManNAz is specifically channeled into the sialic acid biosynthetic pathway, making it an excellent tool for selectively labeling sialoglycoconjugates. • Bioorthogonality: The azide group is biologically inert, ensuring highly specific and non-perturbing labeling in complex biological environments. • Versatility: Compatible with various click chemistry reactions (CuAAC and especially SPAAC/copper-free click for live systems), offering flexibility for diverse experimental designs. • Cell Permeability: The acetyl groups facilitate efficient uptake into cells. • Non-Radioactive: A safe and convenient alternative to traditional radioisotope labeling methods. 4. Challenges and Limitations While powerful, Ac4ManNAz also presents certain considerations: • Concentration-Dependent Toxicity: While generally considered safe at optimal concentrations (typically 10-50 μM for cell labeling), higher concentrations of azidosugars can potentially exert off-target effects, alter cellular metabolism, or induce cytotoxicity. Optimization of the labeling concentration for each cell type and experimental condition is crucial. Some studies have noted that 10 μM Ac4ManNAz can show good labeling efficiency with minimal impact on cellular functions. • Efficiency of Incorporation in vivo: The efficiency of sialic acid labeling with Ac4ManNAz can be lower in vivo compared to in vitro cell culture systems. Metabolic bottlenecks and competition with endogenous sugars can impact incorporation levels in different organs. • Metabolic Feedback and Perturbations: While metabolic glycoengineering aims to be non-perturbing, the introduction of unnatural sugar analogs can, in some cases, induce subtle changes in metabolic flux or alter the overall glycosylation profile if used at very high concentrations or for extended periods. • Copper Toxicity (for CuAAC): As with Ac4GalNAz, if copper-catalyzed click chemistry (CuAAC) is employed, the cytotoxicity of copper ions must be managed, often necessitating the use of copper-free click reagents for live-cell or in vivo experiments. • Potential for Non-Enzymatic Labeling: In some instances, very high doses of azidosugars might lead to minor non-enzymatic labeling of proteins, though this is less common with optimal concentrations and careful experimental design. 5. Ac4ManNAz vs. Ac4GalNAz (Specificity Comparison) It’s important to distinguish between Ac4ManNAz and Ac4GalNAz in terms of their metabolic specificity: • Ac4ManNAz: Primarily targets the sialic acid biosynthetic pathway. After deacetylation to ManNAz, it is converted to SiaNAz and incorporated into sialylated glycoconjugates (e.g., N-glycans, O-glycans, glycolipids that terminate with sialic acid). • Ac4GalNAz: Primarily targets the mucin-type O-linked glycosylation pathway. After deacetylation to GalNAz, it is converted to UDP-GalNAz and incorporated as the initiating sugar (core GalNAc) of mucin-type O-linked glycoproteins. While both are powerful bioorthogonal reporters, their utility lies in targeting distinct glycosylation pathways. It’s worth noting that metabolic cross-talk can occur. For instance, GalNAz can be epimerized to GlcNAz, leading to some labeling of N-glycans and O-GlcNAc. Similarly, ManNAz is generally considered very specific for sialic acid. Ac4ManNAz stands as an indispensable tool in glycobiology, providing a sophisticated means to study the complex world of sialylation. Its ability to introduce a bioorthogonal handle into sialoglycans has enabled significant advancements in understanding cellular communication, disease progression, and the development of novel diagnostics and therapeutics. As research in glycobiology continues to expand, Ac4ManNAz, alongside other metabolic chemical reporters, will remain at the forefront of innovative approaches to probe and manipulate biological systems. References: 1. Ac4ManNAz 2. Physiological Effects of Ac4ManNAz and Optimization of Metabolic Labeling for Cell Tracking 3. Recent advances in developing active targeting and multi-functional drug delivery systems via bioorthogonal chemistry 4. Overview of Click Chemistry |
|
Ac4ManNAz
For Research & Development use only. Not for testing and/or use on humans.