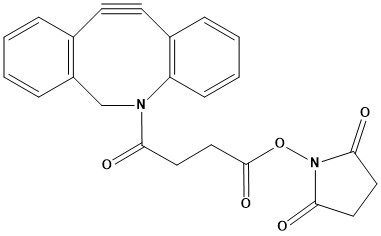
| Synonym: | N-Succinimidyl 4-[(5-Aza-3,4:7,8-dibenzocyclooct-1-yne)-5-yl]-4-oxobutyrate |
| CAS #: | 1353016-71-3 |
| Molecular Formula: | C23H18N2O5 |
| Molecular Weight: | 402.4 |
| DBCO-NHS ester, also known as Dibenzocyclooctyne-N-hydroxysuccinimide ester or sometimes DIBAC-NHS ester, is a highly versatile and widely utilized bioconjugation reagent in modern chemical biology and biotechnology. It is a cornerstone of “click chemistry,” specifically the copper-free variant known as strain-promoted azide-alkyne cycloaddition (SPAAC). 1. Chemical Structure and Properties DBCO-NHS ester features two distinct reactive moieties: • Dibenzocyclooctyne (DBCO) Group: This is a strained alkyne, part of a bicyclic ring system, which allows it to undergo cycloaddition reactions with azides without the need for a copper catalyst. The inherent strain in the alkyne bond makes it highly reactive, facilitating rapid and efficient conjugation under mild conditions. • N-Hydroxysuccinimide (NHS) Ester Group: This is an activated ester that readily reacts with primary amine groups found on biomolecules (e.g., lysine residues in proteins, the N-terminus of peptides, or amine-modified oligonucleotides). This reaction forms a stable amide bond, effectively linking the DBCO moiety to the amine-containing molecule. It is typically supplied as a white to off-white solid and is soluble in organic solvents such as dimethyl sulfoxide (DMSO), dichloromethane (DCM), and dimethylformamide (DMF). It should be stored at -20°C and is sensitive to moisture, light, and heat. 2. Mechanism of Action The utility of DBCO-NHS ester stems from its dual reactivity: • Amine Acylation (via NHS Ester): The NHS ester portion of the molecule reacts selectively with primary amine groups (R-NH2 under slightly alkaline to neutral pH conditions (typically pH 7.2-9). This reaction involves the nucleophilic attack of the amine on the carbonyl carbon of the NHS ester, leading to the displacement of N-hydroxysuccinimide and the formation of a stable amide bond. This step effectively introduces the DBCO group onto the target biomolecule. R-NH2+ DBCO-NHS Ester → R-NH-CO-DBCO + NHS • Strain-Promoted Azide-Alkyne Cycloaddition (SPAAC): Once the DBCO group is attached to a biomolecule, it becomes a “handle” for subsequent conjugation via SPAAC. The strained alkyne of DBCO reacts with an azide (R’-N$_{3}$) on a second molecule. This cycloaddition proceeds spontaneously and rapidly without the need for a metal catalyst (unlike copper-catalyzed click chemistry), forming a stable 1,2,3-triazole linkage. DBCO-labeled Molecule + Azide-labeled Molecule → Conjugate (via triazole) This two-step approach allows for orthogonal bioconjugation, where the amine-reactive step is performed first, followed by the highly specific and bioorthogonal SPAAC reaction with an azide-modified target. 3. Key Applications DBCO-NHS ester is an indispensable tool across various fields of life science and materials science: • Bioconjugation: Its primary application is the conjugation of biomolecules such as proteins, antibodies, peptides, and nucleic acids. It allows researchers to site-selectively introduce the DBCO handle onto amine-containing biomolecules for subsequent click reactions. • Antibody-Drug Conjugates (ADCs): DBCO-NHS ester is frequently employed as a linker in ADC synthesis. It enables the attachment of cytotoxic payloads (which are often azide-modified) to antibodies that have been pre-functionalized with DBCO groups via their lysine residues. This strategy is crucial for targeted drug delivery in cancer therapy. Some DBCO-NHS ester variants may include a cleavable linker (e.g., disulfide bond) for intracellular release of the payload. • Surface Modification: It is widely used to functionalize surfaces (e.g., glass slides, nanoparticles, biosensors, microfluidic devices) with DBCO groups, allowing for the immobilization of azide-tagged biomolecules. This is vital for diagnostic assays, biosensor development, and creating bio-interfaces. • Cellular Labeling and Imaging: DBCO-NHS ester can be used to label cell surface proteins or other biomolecules that have been metabolically engineered to display azide groups. This enables imaging of specific cellular components without interfering with cellular processes, as the SPAAC reaction is biocompatible and proceeds under physiological conditions. • Hydrogel Formation: It can be used to create hydrogels by crosslinking polymers functionalized with amines and then reacting them with azide-modified polymers or biomolecules. • PROTAC Synthesis: In proteolysis-targeting chimeras (PROTACs), DBCO-NHS ester can serve as a linker to connect different modules (e.g., a binder for an E3 ligase and a binder for a target protein) for targeted protein degradation. 4. Advantages of DBCO-NHS Ester • Copper-Free Reaction: The most significant advantage is the elimination of cytotoxic copper catalysts, making SPAAC highly suitable for in vivo and in cellulo applications where copper toxicity would be problematic. • High Reactivity: DBCO is one of the most reactive cycloalkynes for SPAAC, ensuring efficient and rapid conjugation with azides. • High Chemoselectivity: The DBCO-azide reaction is highly specific and does not react or interfere with most other common functional groups found in biological systems, allowing for precise modification. • Biocompatibility: The reaction proceeds under mild physiological conditions (aqueous buffers, room temperature, neutral pH), preserving the integrity and activity of sensitive biomolecules. • Versatility: The NHS ester allows for easy attachment to a wide range of amine-containing molecules, providing a universal handle for subsequent click chemistry. • Orthogonal Chemistry: It enables a two-step conjugation strategy, where the DBCO handle is first introduced, followed by the specific azide-mediated click reaction, offering precise control over the modification. 5. Considerations for Use While highly advantageous, researchers should be aware of a few considerations when working with DBCO-NHS ester: • Hydrolysis of NHS Ester: Like all NHS esters, DBCO-NHS ester is susceptible to hydrolysis in aqueous solutions, especially at higher pH. Therefore, stock solutions should be prepared in dry organic solvents (like DMSO or DMF) immediately before use and reactions with amines should ideally be performed relatively quickly. • Solubility: While the NHS ester allows conjugation in aqueous buffers, the DBCO core itself is relatively hydrophobic. For some applications, particularly when dealing with large biomolecules or requiring higher aqueous solubility, PEGylated versions of DBCO-NHS ester (e.g., DBCO-PEG-NHS ester) are available to improve solubility and reduce aggregation. • Steric Hindrance: The bulky nature of the DBCO group can sometimes introduce steric hindrance, potentially affecting the binding or activity of conjugated biomolecules. However, this is often mitigated by optimized linker lengths (e.g., using PEG spacers). • Storage: Proper storage at low temperatures (-20°C) in a dry, dark environment is crucial to maintain its reactivity and stability over time. DBCO-NHS ester is an invaluable and powerful reagent for researchers engaged in bioconjugation, drug delivery, diagnostics, and materials science. Its combination of an amine-reactive NHS ester with the highly efficient and bioorthogonal DBCO group makes it a go-to choice for creating complex molecular architectures without the need for toxic catalysts. As click chemistry continues to evolve, DBCO-NHS ester remains a central player, enabling new frontiers in synthetic biology and biomedical applications. References 1. Overview of Click Chemistry 2. Click chemistry 3. Copper-free click chemistry 4. Introduction: Click Chemistry 5. Click Chemistry Azide-Alkyne Cycloaddition |
|
DBCO-NHS Ester
For Research & Development use only. Not for testing and/or use on humans.