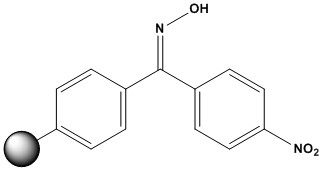
| Synonym: | 4-Nitro Benzophenone Oxime Resin |
| Cross Linker (DVB): | 1% |
| Particle Size (mesh): | 100-200 |
| Loading (mmol/g): | 1.0-1.5 |
| Oxime resins have been used for the synthesis of carboxylic acid derivatives by nucleolytic cleavage [1,2]. Carboxylic acids can be attached to the resin using DCC method. As the oxime linkage is not completely stable to TFA, the use of 25% TFA in DCM is recommended for removal of Boc groups. Caboxamides [3,4], esters [1,2], and enantiopure hydroxamic acids [5] have been synthesized with the resin and using hydrazine and amines, amino acid esters, and hydroxylamine, respectively, as cleavage agents. N-Methyloctadepsipeptides attached to the resin were cyclized by heating them in refuxing ethyl acetate for 2 days to give cyclodepsipeptide PF1022A analogues [6]. References [1] W. F. DeGrado & E. T. Kaiser, J. Org. Chem., 1980, 45, 1295 [2] W. F. DeGrado & E. T. Kaiser, J. Org. Chem., 1982, 47, 3258 [3] N. Vyer, et al., Tetrahedron Lett., 1994, 35, 355 [4] W. C. Lumma, et al., J. Med. Chem., 1998, 41, 1011 [5] E. Thouin & W. D. Lubell, Tetrahedron Lett., 2000, 41, 457 [6] B. H. Lee, et al., Bioorg. Med. Chem. Lett., 2002, 12, 353 |
|
Oxime Resin
(100-200 mesh, 1.0-1.5 mmol/g)
For Research & Development use only. Not for testing and/or use on humans.