| Cross Linker (DVB): | 1% |
| Particle Size (mesh): | 200-400 |
| Loading (mmol/g): | 0.3-1.0 |
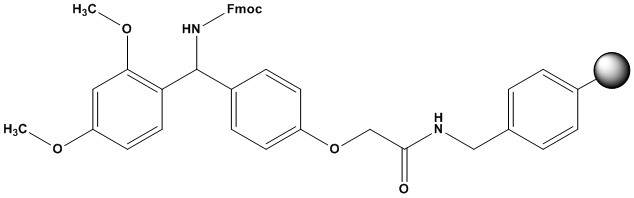
| Rink Amide-AM Resin, often referred to as Fmoc-Rink Amide AM resin, is a cornerstone solid-phase support widely utilized in Fmoc-based solid-phase peptide synthesis (SPPS). It is specifically designed for the efficient synthesis of C-terminal peptide amides, which are prevalent in biologically active peptides and many drug candidates. 1. Chemical Structure and Properties • CAS Number: 183599-10-2 • Synonyms: 4-(2′,4′-Dimethoxyphenyl-Fmoc-aminomethyl)phenoxyacetamidoaminomethyl resin. • Matrix: Typically composed of a polystyrene-divinylbenzene (PS-DVB) copolymer, usually 1% DVB cross-linked, forming spherical beads. This matrix provides mechanical stability, chemical inertness, and good swelling properties in common SPPS solvents. • Linker: The resin incorporates the Rink amide linker, which is a highly acid-labile linker. The “AM” (aminomethyl) designation typically refers to the resin to which the Rink amide linker is attached, often an aminomethyl polystyrene resin. • Functional Group: The resin is pre-functionalized with an Fmoc-protected primary amine group, ready for the coupling of the first amino acid. • Appearance: Pale white or slight yellow beads. • Loading Capacity (Substitution): Typically ranges from 0.4 to 1.0 mmol/g, indicating the amount of Fmoc-amino group available per gram of resin. This is a crucial parameter for determining the scale of peptide synthesis. • Mesh Size: Commonly available in 100-200 mesh or 200-400 mesh, referring to the bead size. Smaller mesh sizes (larger mesh numbers) offer higher surface area and faster reaction kinetics but can lead to higher backpressure in automated synthesizers. • Swelling Properties: Exhibits excellent swelling in common SPPS solvents like DMF (dimethylformamide), DCM (dichloromethane), NMP (N-methyl-2-pyrrolidone), and to some extent, even water. Good swelling is critical for reagents to access the reactive sites within the polymer matrix. • Storage: Generally recommended to store at room temperature (RT) or 2-8°C, often under inert gas to prevent degradation. 2. Mechanism in Fmoc SPPS The functionality of Rink Amide-AM Resin is intrinsically linked to the Fmoc SPPS strategy: • Fmoc Deprotection of Resin: Before the first amino acid coupling, the Fmoc protecting group on the resin’s terminal amine is removed using a mild base, typically a 20% solution of piperidine in DMF. This generates a free amine group on the resin. • First Amino Acid Coupling: The C-terminal amino acid (Fmoc-protected at its alpha-amine) is coupled to the deprotected amine of the Rink Amide linker on the resin. This forms the first amide bond. Standard coupling reagents (e.g., DIC/HOBt, HBTU, HATU) are used. • Iterative Peptide Elongation: The Fmoc protecting group of the coupled amino acid is then removed, exposing a new free amine. This process is repeated, adding one amino acid at a time, to build the desired peptide sequence from the C-terminus to the N-terminus. Side-chain protecting groups (e.g., Boc, tBu, Pbf, Trt) remain intact throughout this process. • Cleavage of Peptide Amide: Once the full peptide sequence is assembled, the peptide is cleaved from the resin. Rink Amide-AM resin is designed for acidic cleavage. This is typically achieved using a solution of trifluoroacetic acid (TFA), usually 90-95% TFA, often with scavengers (e.g., triisopropylsilane (TIS), water, 1,2-ethanedithiol) to prevent reattachment of side-chain protecting groups or other undesirable side reactions during cleavage. The cleavage simultaneously removes all acid-labile side-chain protecting groups. The final product is a peptide with a C-terminal amide. 3. Advantages of Rink Amide-AM Resin • C-terminal Amide Formation: Its primary advantage is its ability to directly synthesize peptides with a C-terminal amide group (CONH2), which is common in many natural and synthetic biologically active peptides (e.g., hormones, neuropeptides). • Fmoc Compatibility: It is perfectly suited for Fmoc chemistry, which is widely favored due to its mild deprotection conditions (piperidine, a weak base) that are less likely to cause racemization or side reactions compared to Boc chemistry. • High Yields and Purity: When used correctly, it facilitates the synthesis of peptides in high yields and purities, a critical factor for downstream applications in drug discovery and basic research. • Acid-Labile Cleavage: The cleavage from the resin is achieved under relatively mild acidic conditions (95% TFA), which also removes most common acid-labile side-chain protecting groups in one step. • Robust Linker: The Rink amide linker, particularly its “AM” variant, is generally more stable to side reactions (e.g., diketopiperazine formation) than some other amide resins, especially for shorter peptides. • Good Swelling Properties: Ensures efficient diffusion of reagents throughout the resin beads, leading to higher reaction completeness. • Versatile: Compatible with a wide range of coupling reagents and solvents used in SPPS. 4. Applications Rink Amide-AM resin is indispensable in various fields: • Peptide Synthesis: The cornerstone application, allowing researchers to synthesize a vast array of linear and modified peptide amides. • Drug Discovery and Development: Crucial for the synthesis of therapeutic peptides, including those used in drug discovery, clinical trials, and commercial production (e.g., peptide hormones, antimicrobial peptides, enzyme inhibitors). • Bioconjugation: Provides a platform for attaching peptides to other biomolecules (e.g., proteins, nanoparticles, polymers) for developing targeted drug delivery systems, diagnostic tools, and vaccine candidates. • Protein Engineering: Used in the modification of proteins or the synthesis of peptide fragments for protein ligation strategies. • Academic Research: A fundamental tool in academic laboratories for teaching and research in peptide chemistry, biochemistry, and medicinal chemistry. • Material Science: Used in the synthesis of peptide-based materials or peptide-functionalized surfaces. 5. Comparison with Other Resins • Rink Amide MBHA Resin: Another common Rink Amide variant. Rink Amide-AM resin is built on an aminomethyl polystyrene matrix, while Rink Amide-MBHA (Methylbenzhydrylamine) is built on an MBHA resin. Both provide C-terminal amides, but subtle differences in acid lability and side reaction profiles can exist. Rink Amide-AM is generally considered a standard workhorse. • Wang Resin/Merrifield Resin: These resins yield C-terminal carboxylic acids after cleavage (Wang is Fmoc-compatible, Merrifield is Boc-compatible). Rink Amide-AM resin is chosen specifically when a C-terminal amide is required. • Sieber Amide Resin: Offers a more acid-sensitive cleavage (e.g., 1% TFA in DCM), allowing for the cleavage of protected peptide amides, which is useful for subsequent solution-phase modifications. Rink Amide-AM generally requires stronger acid for full cleavage and global deprotection. • 2-Chlorotrityl Chloride Resin: Extremely acid-labile. Primarily used for synthesizing protected peptide fragments or peptides with sensitive side chains, as cleavage occurs under very mild acidic conditions (e.g., 1% TFA in DCM), leaving most side-chain protecting groups intact. Rink Amide-AM Resin is an indispensable and highly reliable solid-phase support for the synthesis of C-terminal peptide amides using Fmoc chemistry. Its optimized linker design, compatibility with standard SPPS protocols, and clean acid-mediated cleavage make it a preferred choice for researchers and industrial practitioners in various fields, from fundamental peptide chemistry to the development of complex peptide therapeutics. Its consistent performance, high loading capacity, and excellent swelling properties solidify its position as a go-to resin for efficient and high-purity peptide synthesis. References: 1. Overview of Peptide Synthesis 2. Fmoc Solid Phase Peptide Synthesis 3. Boc Solid Phase Peptide Synthesis 4. Resins for Solid Phase Peptide Synthesis: Application Guide |
|
Rink Amide-AM Resin
(200-400 mesh, 0.3-1.0 mmol/g)
For Research & Development use only. Not for testing and/or use on humans.