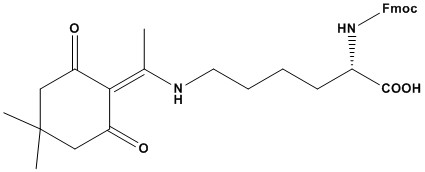
| Synonym: | N-α-Fmoc-N-ε-1-(4,4-dimethyl-2,6-dioxocyclohex-1-ylidene)ethyl-L-lysine |
| CAS #: | 150629-67-7 |
| Molecular Formula: | C31H36N2O6 |
| Molecular Weight: | 532.6 |
| Fmoc-Lys(Dde)-OH is quasi-orthogonally-protected Lys derivative. The Fmoc group can be removed selectively by treatment with piperidine; the Dde groupan be cleaved by 2% hydrazine in DMF. When removing Dde in the presence of allyl based protecting groups, allyl alcohol should be included in the deprotection solution to prevent reduction of the allyl group. Lys(Dde) has been employed in the following applications: synthesis of branched peptides and di-epitopic peptides; preparation of MAP core molecules and lipo-MAPs;the synthesis of cyclic peptides, TASP molecules, templates for combinatorial chemistry and synthetic proteins; preparation of peptides modified at the lysine side-chain. References 1. B. W. Bycroft, et al. (1993) J. Chem. Soc., Chem. Commun., 778. 2. B. Rohwedder, et al. (1998) Tetrahedron Lett., 39, 1175. 3. N. Ahlborg (1995) J. Immun. Meth., 179, 269. 4. B. W. Bycroft, et al. in “Peptides, Chemistry, Structure & Biology, Proc. 13th American peptide Symposium”, R. S. Hodges & J. A. Smith (Eds), ESCOM, Leiden, 1994, pp. 727. 5. J. Mack, et al. (2001) J. Peptide Sci., 7, 338. 6. G. B. Bloomberg, et al. (1993) Tetrahedron Lett., 34, 4709. 7. P. Dumy, et al. (1995) Tetrahedron Lett., 36, 1255. 8. J. Eichler, et al. (1994) Pept. Res., 7, 300. 9. C. G. Fields, et al. (1993) Biopolymers, 33, 1695. 10. H. F. Brugghe, et al. (1994) Int. J. Peptide Protein Res., 43, 166. 11. P. Hoogerhout, et al. (1995) Infection & Immunity, 63, 3473. 12. D. Lelievre, et al. (1995) Tetrahedron Lett., 36, 9317. 13. P. Hoogerhout, et al. (1999) J. Peptide Res., 54, 436. 14. P.J. Conolly, et al. (2000) Tetrahedron Lett., 41, 5187. |
|
Fmoc-Lys(Dde)-OH
For Research & Development use only. Not for testing and/or use on humans.